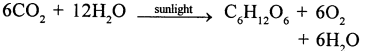
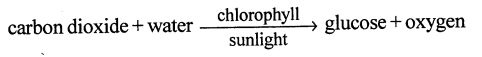
Selina Concise Chemistry Class 6 ICSE Solutions – Elements, Compounds, Symbols and Formulae
ICSE SolutionsSelina ICSE SolutionsML Aggarwal Solutions
APlusTopper.com provides step by step solutions for Selina Concise ICSE Solutions for Class 6 Chemistry. You can download the Selina Concise Chemistry ICSE Solutions for Class 6 with Free PDF download option. Selina Publishers Concise Chemistry for Class 6 ICSE Solutions all questions are solved and explained by expert teachers as per ICSE board guidelines.
Selina Class 6 Chemistry ICSE SolutionsPhysicsBiologyMathsGeographyHistory & Civics
Selina Concise ICSE Solutions for Class 6 Chemistry Chapter 4 Elements, Compounds, Symbols and Formulae
POINTS TO REMEMBER
- Pure substances : “A single substance of definite composition.” Pure substances are homogeneous. They are made up of only one kind of atoms and compounds or made up of only one kind of molecules.
- Elements : An element is defined as a pure substance made up of only one kind of atoms that cannot be converted into anything simpler than itself by any physical or chemical process.
- Metals : Most of the elements known to us are metals. Example: Sodium potassium, iron, gold, silver are elements. Metals are lustrous, hard, ductile and malleable, good conductor of electricity. Mercury (liquid) have High M.P. and High B.P. but exceptions are there.
- Non-metals : Carbon, phosphorous, sulphur all solids, Bromine (liquid) iodine(s) and rest are gases, dull, do not shine. Cannot be drawn into wires, non malleable, have low M.P. and B.P. do not produce sound when struck, do not conduct electricity. Exceptions are there.
- Boron, sillicon, arsenic, antimony resemble both metals and non-metals and are called metalloids.
- Unreactive gases, also called noble gases are gases which are very unreactive i.e. He, Ne Ar, Kr, xenon and radon.
- Symbols are abbreviations that are used to denote a chemical element which is usually first letter of its name in English or Latin.
- O is the symbol of element oxygen.
C is symbol of element carbon.
Cu is symbol of element copper (taken from Latin name Cuprum) - Compound is formed by the chemical combination of two or more elements in definite ratio (by mass).
- Molecule is the smallest unit of a compound.
- Elements are the basic substances from which all other substances are made.
- Compound : Consist of two or more elements combined in definite proportion, pure and homogeneous, physical and chemical properties are entirely new and different from its consititutent elements, energy is either needed or produced when a compound is formed.
- Atoms : Atom is the smallest unit of an element which cannot be further broken into simpler parts, may or may not have independent existence.
- Molecule of element : When two or more atoms of the same element combine it forms a molecule of an atom. e.g. N2,O2
- Molecule of compounds : When atoms of two or more element combine, they form a molecule of a compound. H20, HC1, CuSO4.
EXERCISE-I
Question 1.
Classify the following substances into elements and compounds.
Answer:
Mercury, sulphur, sugar, water, sand, gold, coal, oxygen, alcohol.
Ans. Element: Mercury, sulphur, gold, coal, oxygen.
Compound : Sugar, water, sand, alcohol.
Question 2.
Give the symbols of: Carbon, calcium, copper, chlorine, cobalt, argon.
Answer:
Carbon is C Chlorine is Cl
Calcium is Ca Cobalt is Co
Copper is Cu Argon is Ar
Question 3.
Define a pure substance. How many types of pure substances do you know ?
Answer:
Pure substances : “A substance of a definite composition which has consistent properties throughout, is called a pure substance”
Types of pure substances: Pure substances are of two types (i) Elements, (ii) Compounds.
Question 4.
Define : (a) Elements (b) Compounds.
Name the particles from which elements and compounds are made of.
Answer:
(a) Elements : An element is defined as a pure substance made up of only one kind of atoms that cannot be converted into anything simpler than itself by any physical or chemical process.
(b) Compounds : Compounds are pure substances composed of two or more elements in definite proportion by mass and has a definite set of properties. Compound is made up of only one kind of molecules.
Question 5.
Give two examples for each of the following :
(a) Metals
(b) Non-metals
(c) Metalloids
(d) Noble gases
Answer:
(a) Metals : Iron, silver, gold.
(b) Non-metals : Carbon, sulphur, oxygen.
(c) Metalloids : Antimony, silicon, boron.
(d) Noble gases : Helium, argon, neon.
Question 6.
Name the elements which form water. How will you justify that water is a compound ?
Answer:
The elements which form water are (i) Hydrogen and oxygen.
Justification : Water has entirely different properties (i.e. is a liquid, extinguishes fire) from the elements it is made up of i.e. Hydrogen a gas catches fire oxygen a gas supporter of combustion.
- Energy is needed to form water on combining O2 with H2.
- We can not seperate the constituents of water by simple physical means.
Question 7.
Give three differences between metals and non-metals.
Answer:
Metals
- Metals are ductile i.e. can be drawn into wires.
- Metals are malleable i.e. can be beaten to form sheets.
- They are sonorous.
Non-metals
- Non-metals are mostly soft solids cannot be drawn into wires.
- They are mostly gases and are not malleable.
- They donot produce sound when struck.
Question 8.
How is sodium chloride different from its constituent elements, sodium and chlorine ?
Answer:
Sodium is a metal that is stored in kerosene oil as it reacts very fast with air and water. Chlorine is a reactive greenish yellow gas which is poisonous. When these two elements combine chemically they form common salt sodium chloride which is non poisonous colourless solid substance that we use in our food to add taste and to obtain some nutrition.
Question 9.
State four important characteristics of compounds.
Answer:
- When compound is formed energy like heat, light or electricity is either needed or produced.
- A compound has properties entirely different from the properties of its constituents.
- Change in weight takes place.
- It cannot be separated into its constituents by simple physical means.
Question 10.
Give two examples for each of the following :
(a) Non-metals which are solids
(b) Metals which are soft
(c) Non-metals which are lustrous
(d) Elements which are liquids.
(e) Inert gases
(f) Metalloids
Answer:
(a) Phosphorus, Sulphur
(b) Lead and Sodium
(c) Radium, Graphite
(d) Mercury, Bromine
(e) Helium, Neon
(f) Antimony, Arsenic
Question 11.
Name the elements present
(a) Sugar
(b) Ammonia
(c) Marble
(d) Washing soda
Answer:
Compounds
(a) Sugar
(b) Ammonia
(c) Marble
(d) Washing soda
Elements present
(a) Carbon, hydrogen & oxygen
(b) Nitrogen and hydrogen
(c) Calcium, carbon & oxygen
(d) Sodium, carbon & oxygen
Question 12.
What is the proportion of elements present in the following compounds?
(a) H2O
(b) CO2
(c) CaO
(d) NO2
Answer:
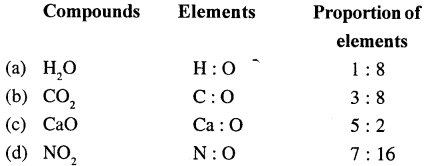
Question 13.
Name two compounds which dissolve in water.
Answer:
Two compounds which dissolve in water are sugar, table salt.
EXERCISE-II
(ATOMS & MOLECULES)
Question 1.
Define:
(a) Atom
(b) Molecule
(c) Atomicity
(d) Formula
Answer:
(a) Atom : An atom is the smallest indivisible unit of an element which exhibits all the properties of that element and may or may not have independent existence.
(b) Molecule : A molecule can be defined as the smallest unit of an element or a compound which exhibits all the properties of that element or compound and has independent existence. They are divisible into atoms.
(c) Atomicity : The number of atoms in a molecule of an element is called its atomicity.
(d) Formula : Formula is a short way of representing the molecule of an element or a compound.
Question 2.
Why are symbols and formulae of substances important?
Answer:
Importance of symbols and Formulae :
Symbols and formulae of substance gives a lot of information like.
- Types of elements present in the compound. E.g. (H20 is made of two elements hydrogen and oxygen).
- Number of each kind of atoms in one molecule. E.g. (water has 2 atoms of hydrogen combined with 1 atom of oxygen.)
- Mass of one molecule of the compound. E.g. [H2O has mass (1 × 2) + 16 = 18 g].
Question 3.
Mention three gaseous elements and write their molecular formulae.
Answer:
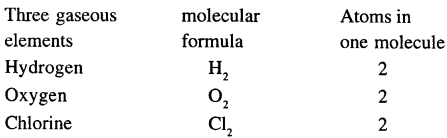
Question 4.
State the informations obtained from the formula of a compound.
Answer:
A formula gives us the following information about a compound.
- Types of elements present in the compound.
- Number of each kind of atoms in one molecule of the compound.
- Mass of one molecule of the compound.
Example:
A molecule of carbon dioxide gas is represented by CO2 It indicates that a carbon dioxide molecule is formed by the combination of two elements i.e. carbon and oxygen. The number of carbon atom is one and that of oxygen atom is two. The mass of one molecule of carbon dioxide can be calculated by adding the mass of one atom of carbon and two atoms of oxygen.
Question 5.
What is meant by
(a) 2H and H2
(b) H20 and 3H2O ?
Answer:
(a) 2H is two atoms of hydrogen. H2 is one molecule of hydrogen gas.
(b) H20 represents one molecule of water. 3H20 represents 3 molecules of water.
Question 6.
State the number of atoms of each kind, present in
(a) C6 H12O6
(b) H2SO4
(c) HNO3
(d) CaCO3
Also name these compounds.
Answer:

Question 7.
Write the molecular formulae of compounds calcium oxide, hydrogen sulphide, carbon monoxide and lead sulphide.
Answer:
Compound Calcium oxide is formed of elements calcium (Ca) and oxygen (O)
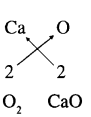
Symbols combining power Here subscript number is same Ca2 Formula of calcium oxide is CaO Compounds Hydrogen sulphide is formed of elements

EXERCISE-III
Question 1.
Name:
(a) Three different forms of carbon.
(b) A form of carbon used as a gem.
(c) Two substances used to make electric wires.
(d) Two substances used to make jewellery.
(e) A substances used as an insulator.
Answer:
(a)
- Diamond
- Graphite
- Coal
(b) Diamond is used as gem.
(c)
- Copper.
- Aluminium as these are good conductors of electricity.
(d)
- Gold.
- Silver as these are shining, lustrous, and ductile.
(e) Plastic is used as insulator as it is bad conductor of electricity.
Question 2.
Give one use of each of the following substances :
(a) Iron
(b) Brass
(c) Coal
Answer:
(a) Iron : To make machines tools and building material.
(b) Brass : To make water taps and utensils.
(c) Coal: Coal is used as fuel also used in thermal power plant to produce electricity.
Question 3.
Give reason:
(a) A frying pan is made up of steel but its handle is made up of wood.
(b) Graphite is used to make lead of the pencils.
(c) Argon is filled in electric bulbs.
Answer:
(a) Steel is good conductor of heat to cook food, pan is made of steel where as wood is insulator of heat and to hold, handle is made up of wood.
(b) Graphite leaves mark on the paper and makes it black.
(c) Argon is inert gas and protects the element of bulb from oxidation and burning. Hence increases bulb’s life.
Question 4.
Answer the following questions:
(a) Why are copper and aluminium used to make electric wires?
(b) What do you understand by the statement: ‘metals are ductile and malleable’?
(e) Give the advantages of using symbols instead of names of elements or compounds.
(d) When iron is mixed with sulphur at room temperature, it does not form a compound. Why?
(e) Find the atomicity of the Tollowing molecules:
- calcium chloride
- aluminium suiphide
- acetic acid
- dinitrogen oxide
- carbon monoxide
Answer:
(a) Copper and aluminium are good conductors of heat and electricity. They can be drawn into wires and beaten into sheets. Therefore, they are used to make electric wires.
(b) Metals are ductile, i.e., they can be drawn or stretched into thin wires. They are malleable, i.e., they can be beaten into thin sheets.
(c)
- Symbols increases scientific communication across the world.
- Symbols helps to make equations and data shorter and concise.
- Symbols are helpful for Scientists as it would take time and paper to do their job. They had to write out the full name of every element instead of its symbol.
(d) When iron is mixed with sulphur at room temperature, it does not form a compound because the mixture of iron and sulphur requires heat to form a compound i.e. iron sulphide.
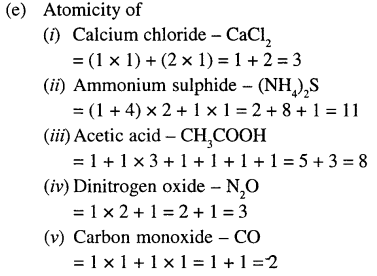
OBJECTIVE TYPE QUESTIONS
1. Fill in the blanks
(a) Atomicity refers to the number of atoms in the molecule of an element.
(b) The most abundant element in the earth’s crust is oxygen.
(c) A metal which is a liquid at room temperature is mercury.
(d) The most abundant element in the atmosphere is nitrogen.
(e) A metal which is a poor conductor of electricity is tungsten.
(f) A diatomic gaseous element is oxygen.
(g) A liquid non-metal is bromine.
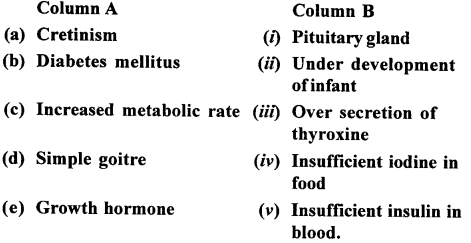
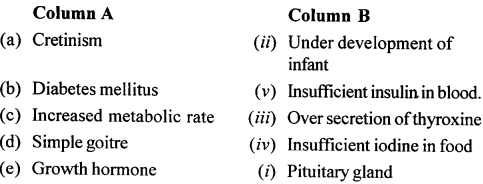
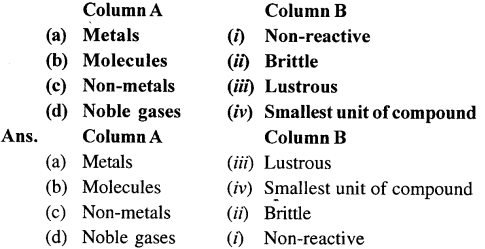
2. Match the columns
3. Indicate whether the following statements are true or false.
(a) A compound is made up of just one kind of atom.
Answer. True False
Correct : A compound is made up of two or more elements is a fixed proportion by mass.
(b) Metals reflect light and are good conductors of electricity.
Answer. True
(c) Metals can be polished.
Answer. True
(d) Elements are made up of compounds.
Answer. False
Correct : Elements are made up of atoms.
(e) All elements are artificially prepared.
Answer. False
Correct: All elements are made up of a limited number of basic substances.
(f) Molecules can exist independently.
Answer. True
(g) Molecules combine to form atoms.
Answer. False
Correct : Atoms combine to form molecule.
(h) Noble gases are high reactive.
Answer. False
Correct: Noble gases are non-reactive.
(i) Ozone is a triatomic molecule.
Answer. True
MULTIPLE CHOICE QUESTIONS
Tick (√) the correct alternative from the choice given for the following statements :
1. All pure substances have
- the same physical state .
- the same colour
- the same composition
- a definite set of properties
2. Sugar is a compound which consists of the elements
- carbon and hydrogen
- hydrogen and oxygen
- carbon, hydrogen and oxygen
- hydrogen, carbon and sulphur
3. Atoms of different kinds combine to form molecules of
- an element
- a compound
- a mixture
- all of the above
4. Sulphur and carbon are
- metals
- non-metals
- metalloids
- noble gases
5. Gold is used to make jewellery because
- it is dull
- lustrous and attractive
- highly reactive
- very cheap
6. The most abundant elements in the universe are
- neon and argon
- hydrogen and helium
- aluminium and copper
- oxygen and nitrogen
7. The compound used as common salt is
- sodium chloride
- calcium chloride
- sodium oxide
- hydrogen chloride
8. Brass and bronze are
- elements
- mixtures
- compounds
- all of the above
9. Sand is a compound of
- silicon and nitrogen
- silicon and oxygen
- oxygen and sulphur
- none of the above
10. From the list given below select the correct substance which is most suitable to the statements given : (oxygen, diamond, zinc, graphite, gold)
- A metal which is brittle.
- A non-metal which is a good conductor of electricity.
- The hardest naturally occurring substance.
- The most ductile metal.
- A gaseous non-metal.
Answer.
(a) Zinc
(b) Graphite
(c) Diamond
(e) Oxygen