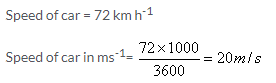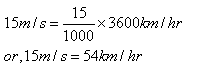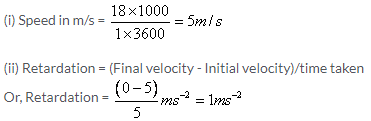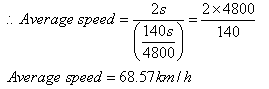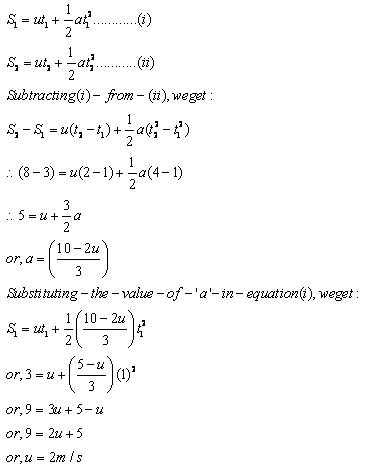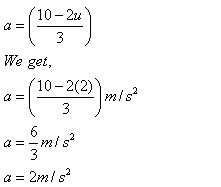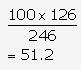Selina Concise Physics Class 9 ICSE Solutions Motion in One Dimension
ICSE SolutionsSelina ICSE Solutions
APlusTopper.com provides step by step solutions for Selina Concise ICSE Solutions for Class 9 Physics Chapter 2 Motion in One Dimension. You can download the Selina Concise Physics ICSE Solutions for Class 9 with Free PDF download option. Selina Publishers Concise Physics for Class 9 ICSE Solutions all questions are solved and explained by expert teachers as per ICSE board guidelines.
Download Formulae Handbook For ICSE Class 9 and 10
Selina ICSE Solutions for Class 9 Physics Chapter 2 Motion in One Dimension
Exercise 2(A)
Solution 1S.
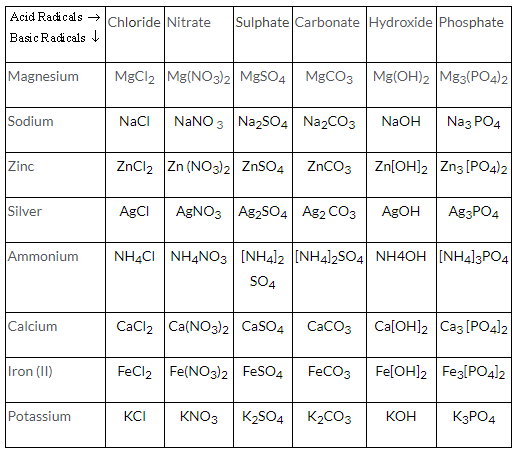
| Scalar | Vector |
| They are expressed only by their magnitudes. | They are expressed by magnitude as well as direction. |
| They can be added, subtracted, multiplied or divided by simple arithmetic methods. | They can be added, subtracted or multiplied following a different algebra. |
| They are symbolically written by English letter. | They are symbolically written by their English letter with an arrow on top of the letter. |
| Example: mass, speed | Example: force, velocity |
Solution 2S.
a) Pressure is a scalar quantity.
b) Momentum is a vector quantity.
c) Weight is a vector quantity.
d) Force is a vector quantity.
e) Energy is a scalar quantity.
f) Speed is a scalar quantity.
Solution 3S.
A body is said to be at rest if it does not change its position with respect to its immediate surroundings.
Solution 4S.
A body is said to be in motion if it changes its position with respect to its immediate surroundings.
Solution 5S.
When a body moves along a straight line path, its motion is said to be one-dimensional motion.
Solution 6S.
The shortest distance from the initial to the final position of the body is called the magnitude of displacement. It is in the direction from the initial position to the final position.
Its SI unit is metre (m).
Solution 7S.
Distance is a scalar quantity, while displacement is a vector quantity. The magnitude of displacement is either equal to or less than the distance. The distance is the length of path travelled by the body so it is always positive, but the displacement is the shortest length in direction from initial to the final position so it can be positive or negative depending on its direction. The displacement can be zero even if the distance is not zero.
Solution 8S.
Yes, displacement can be zero even if the distance is not zero.
For example, when a body is thrown vertically upwards from a point A on the ground, after sometime it comes back to the same point A. Then, the displacement is zero, but the distance travelled by the body is not zero (it is 2h; h is the maximum height attained by the body).
Solution 9S.
The magnitude of displacement is equal to distance if the motion of the body is one-dimensional.
Solution 10S.
The velocity of a body is the distance travelled per second by the body in a specified direction.
Its SI unit is metre/second (m/s).
Solution 11S.
The speed of a body is the rate of change of distance with time.
Its SI unit is metre/second (m/s).
Solution 12S.
Speed is a scalar quantity, while velocity is a vector quantity. The speed is always positive-it is the magnitude of velocity, but the velocity is given a positive or negative sign depending upon its direction of motion. The average velocity can be zero but the average speed is never zero.
Solution 13S.
Velocity gives the direction of motion of the body.
Solution 14S.
Instantaneous velocity is equal to average velocity if the body is in uniform motion.
Solution 15S.
If a body travels equal distances in equal intervals of time along a particular direction, then the body is said to be moving with a uniform velocity. However, if a body travels unequal distances in a particular direction in equal intervals of time or it moves equal distances in equal intervals of time but its direction of motion does not remain same, then the velocity of the body is said to be variable (or non-uniform).
Solution 16S.
Average speed is the ratio of the total distance travelled by the body to the total time of journey, it is never zero. If the velocity of a body moving in a particular direction changes with time, then the ratio of displacement to the time taken in entire journey is called its average velocity. Average velocity of a body can be zero even if its average speed is not zero.
Solution 17S.
The motion of a body in a circular path with uniform speed has a variable velocity because in the circular path, the direction of motion of the body continuously changes with time.
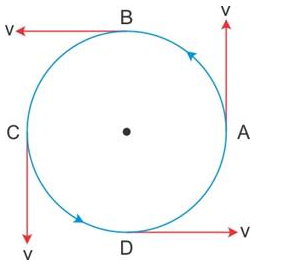
Solution 18S.
If a body starts its motion from a point and comes back to the same point after a certain time, then the displacement is zero, average velocity is also zero, but the total distance travelled is not zero, and therefore, the average speed in not zero.
Solution 19S.
Acceleration is the rate of change of velocity with time.
Its SI unit is metre/second2 (m/s2).
Solution 20S.
Acceleration is the increase in velocity per second, while retardation is the decrease in velocity per second. Thus, retardation is negative acceleration. In general, acceleration is taken positive, while retardation is taken negative.
Solution 21S.
The acceleration is said to be uniform when equal changes in velocity take place in equal intervals of time, but if the change in velocity is not the same in the same intervals of time, the acceleration is said to be variable.
Solution 22S.
Retardation is the decrease in velocity per second.
Its SI unit is metre/second2 (m/s2).
Solution 23S.
Velocity determines the direction of motion.
Solution 24S.
(a) Example of uniform velocity: A body, once started, moves on a frictionless surface with uniform velocity.
(b) Example of variable velocity: A ball dropped from some height is an example of variable velocity.
(c) Example of variable acceleration: The motion of a vehicle on a crowded road is with variable acceleration.
(d) Example of uniform retardation: If a car moving with a velocity ‘v’ is brought to rest by applying brakes, then such a motion is an example of uniform retardation.
Solution 25S.
Initially as the drops are equidistant, we can say that the car is moving with a constant speed but later as the distance between the drops starts decreasing, we can say that the car slows down.
Solution 26S.
When a body falls freely under gravity, the acceleration produced in the body due to the Earth’s gravitational acceleration is called the acceleration due to gravity (g). The average value of g is 9.8 m/s2.
Solution 27S.
No. The value of ‘g’ varies from place to place. It is maximum at poles and minimum at the Equator on the surface of the Earth.
Solution 28S.
In vacuum, both will reach the ground simultaneously because acceleration due to gravity is same (=g) on both objects.
Solution 1M.
Velocity is a vector quantity. The others are all scalar quantities.
Solution 2M.
m s-1
Solution 3M.
m s-2
Solution 4M.
The displacement is zero.
Solution 5M.
5 m s-1
Solution 1N.
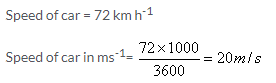
Solution 2N.
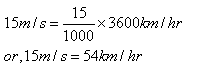
Solution 3N.

Solution 4N.
18 km h-1 < 10 m s-1 < 1 km min-1
Solution 5N.
Total time taken = 3 hours
Speed of the train = 65 km/hr
Distance travelled = speed x time
= 65 x 3 = 195 km
Solution 6N.

Solution 7N.

(ii) Average velocity of the train is zero because the train stops at the same point from where it starts, i.e. the displacement is zero.
Solution 8N.

Solution 9N.
Here, final velocity = 10 m/s
Initial velocity = 0 m/s
Time taken = 2s
Acceleration = (Final Velocity – Initial Velocity)/time
= (10/2) m/s2
= 5 m/s2
Solution 10N.
Here, final velocity = 180 m/s
Initial velocity = 0 m/s
Time taken = 0.05 h or 180 s
Acceleration = (Final Velocity – Initial Velocity)/time
= (180-0)/180 m/s2
= 1 m/s2
Solution 11N.
Here, final velocity = 20 m/s
Initial velocity = 50 m/s
Time taken = 3 s
Acceleration = (Final Velocity – Initial Velocity)/time
= (20 – 50)/3 m/s2
= -10 m/s2
Negative sign here indicates that the velocity decreases with time, so retardation is 10 m/s2.
Solution 12N.
Here, final velocity = 18 km/h or 5 m/s
Initial velocity = 0 km/h
Time taken = 2 s
Acceleration = (Final Velocity – Initial Velocity)/time
= (5 – 0) / 2 m/s2
= 2.5 m/s2
Solution 13N.
Acceleration = Increase in velocity/time taken
Therefore, increase in velocity = Acceleration × time taken
= (5 × 2) m/s
= 10 m/s
Solution 14N.
Initial velocity of the car, u = 20 m/s
Retardation = 2 m/s2
Given time, t = 5 s
Let ‘v’ be the final velocity.
We know that, Acceleration = Rate of change of velocity /time
= (Final velocity – Initial velocity)/time
Or, -2 = (v – 20) / 5
Or, -10 = v – 20
Or, v = -20 + 10 m/s
Or, v = -10 m/s
Negative sign indicates that the velocity is decreasing.
Solution 15N.
Initial velocity of the bicycle, u = 5 m/s
Acceleration = 2 m/s2
Given time, t = 5 s
Let ‘v’ be the final velocity.
We know that, acceleration = Rate of change of velocity/time
= (Final velocity – Initial velocity)/time
Or 2 = (v – 5)/5
Or, 10 = (v – 5)
Or, v = -5 – 10
Or, v = -15 m/s
Negative sign indicates that the velocity is decreasing.
Solution 16N.
Initial velocity of the bicycle, u = 18 km/hr
Time taken, t = 5 s
Final velocity, v = 0 m/s (As the car comes to rest)
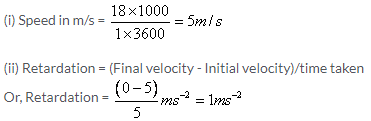
(iii) Let ‘V’ be the speed of the car after 2 s of applying the brakes.
Then, Acceleration = (V – 5)/ 2
Or, -1 = (V – 5)/2
Or, V = -2 + 5
Or, V = 3 m/s2
Exercise 2(B)
Solution 1S.
For the motion with uniform velocity, distance is directly proportional to time.
Solution 2S.
From displacement-time graph, the nature of motion (or state of rest) can be understood. The slope of this graph gives the value of velocity of the body at any instant of time, using which the velocity-time graph can also be drawn.
Solution 3S.
(a) Slope of a displacement-time graph represents velocity.
(b) The displacement-time graph can never be parallel to the displacement axis because such a line would mean that the distance covered by the body in a certain direction increases without any increase in time, which is not possible.
Solution 4S.
(a) There is no motion, the body is at rest.
(b) It depicts that the body is moving away from the starting point with uniform velocity.
(c) It depicts that the body is moving towards the starting point with uniform velocity.
(d) It depicts that the body is moving with variable velocity.
Solution 5S.
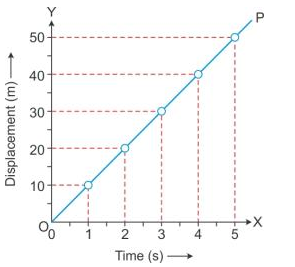
Solution 6S.
(i) The slope of the velocity-time graph gives the value of acceleration.
(ii) The total distance travelled by a body in a given time is given by the area enclosed between the velocity-time graph and X-axis (without any sign).
(iii) The displacement of a body in a given time is given by the area enclosed between the velocity-time graph and X-axis (with proper signs).
Solution 7S.
Vehicle A is moving with a faster speed because the slope of line A is more than the slope of line B.
Solution 8S.
(a) Fig. 4.33 (a) represents uniformly accelerated motion. For example, the motion of a freely falling object.
(b) Fig. 4.33 (b) represents motion with variable retardation. For example, a car approaching its destination.
Solution 9S.
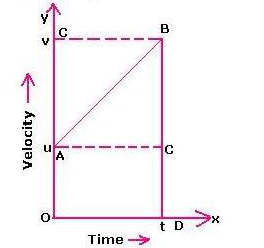
In this graph, initial velocity = u
Velocity at time t = v
Let acceleration be ‘a’
Time = t
Then, distance travelled by the body in t s = area between the v-t graph and X-axis
Or distance travelled by the body in t s = area of the trapezium OABD
= (1/2) × (sum of parallel sides) × (perpendicular distance between them)
= (1/2) × (u + v) × (t)
= (u + v)t /2
Solution 10S.
The slope of the velocity-time graph represents acceleration.
Solution 11S.
Car B has greater acceleration because the slope of line B is more than the slope of line A.
Solution 12S.
For body A: The graph is a straight line. So, the slope gives constant velocity. Hence, the acceleration for body A is zero.
For body B: The graph is a straight line. So, the slope gives constant velocity. Hence, the acceleration for body B is also zero.
For body C: The slope of the graph is decreasing with time. Hence, the acceleration is negative.
For body D: The slope of the graph is increasing with time. Hence, the acceleration is positive.
Solution 13S.
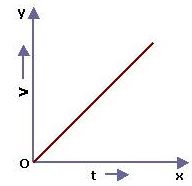
Velocity-time for a body moving with uniform velocity and uniform acceleration.
Solution 14S.
Retardation is calculated by finding the negative slope.
Solution 15S.
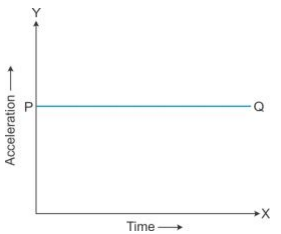
The area enclosed between the straight line and time axis for each interval of time gives the value of change in speed in that interval of time.
Solution 16S.
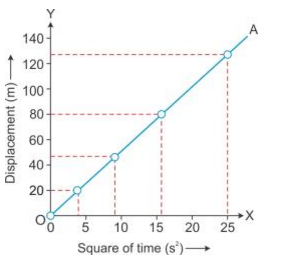
Solution 17S.
For motion under uniform acceleration, such as the motion of a freely falling body, distance is directly proportion to the square of the time.
Solution 18S.
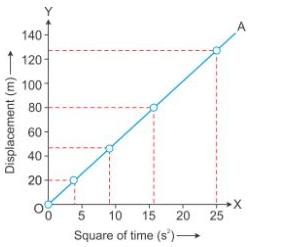
The value of acceleration due to gravity (g) can be obtained by doubling the slope of the S – t2 graph for a freely falling body.
Solution 1M.
B
Acceleration is uniform.
Solution 2M.
The solution is C.
The speed-time graph is a straight line parallel to the time axis.
Solution 3M.
D
A straight line inclined to the time axis.
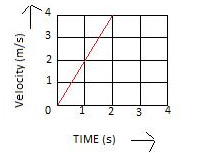
Solution 1N.
Velocity of body at t = 1s is 2 m/s
Velocity of body at t = 2s is 4 m/s
Velocity of body at t = 3s is 6 m/s
Solution 2N.
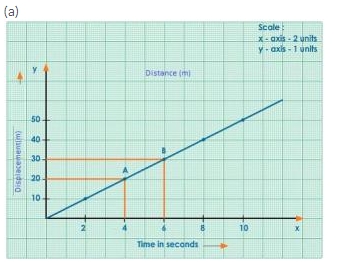
Displacement-time graph
From the part AB of the graph,
Average velocity = (Displacement at B – Displacement at A)/Time taken
= (30 – 20) m/( 6 – 4) s
= (10/2) m/s
= 5 m/s
(b) (i) From the graph, the displacement of car at 2.5 s is 12.5 m.
(ii) From the graph, the displacement of car at 4.5 s is 22.5 m.
Solution 3N.
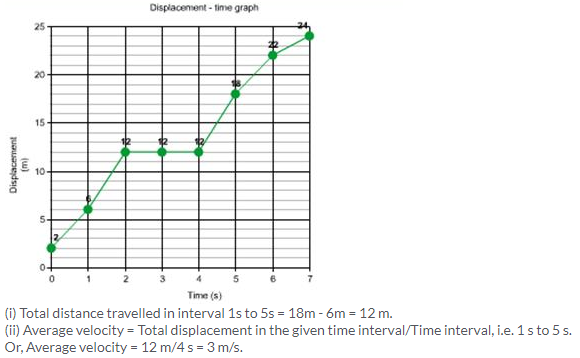
Solution 4N.
(a) (i) Velocity from 0 to 5 s = Displacement /time
= (3/5) m/s
= 0.6 m/s
(ii) Velocity from 5 s to 7 s = Displacement /time
= (0/2) m/s
= 0 m/s.
(iii) Velocity from 7 s to 9 s = Displacement /time
= (7 – 3)/(9 – 7) m/s
= (4/2) m/s
= 2 m/s
(b) From, 5 s to 9 s, displacement = 7m – 3m = 4m.
Time elapsed between 5 s to 9 s = 4 s
Average velocity = Displacement/time
= (4/4) m/s
= 1 m/s
Solution 5N.
(i) Displacement in first 4s = 10 m
Therefore, the average velocity = Displacement/time
= (10/4) m/s
= 2.5 m/s
(ii) Initial position = 0 m
Final position at the end of 10 s = -10m
Displacement = Final position – Initial position
= (-10) m – 0
= -10 m
(iii) At 7 s and 13 s, the cyclist reaches his starting point.
Solution 6N.
(i) Initially, the car B was 40 km ahead of car A.
(ii) Straight line depicts that cars A and B are moving with uniform velocities.
For car A
Displacement at t = 1 h is 40 m
Velocity = Displacement /time
= (40/1) km/h
= 40 km/h
For car B
Displacement at t = 4 h is (120 – 40) km, i.e. 80 km
Velocity = Displacement /time
= (80/4) km/h
= 20 km/h
(iii) Car A catches car B in 2 hours.
(iv) After starting, car A will catch car B at 80 km.
Solution 7N.
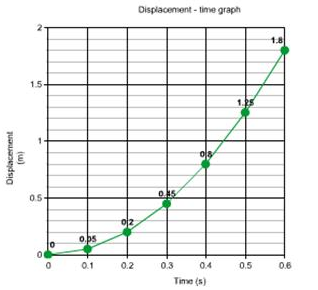
Solution 8N.
Velocity of the body at t = 1 s is 1 m/s.
Displacement of the body at t = 1 s is velocity × time = (1) × (1) m or 1 m.
Velocity of the body at t = 2s is 2 m/s.
Displacement of the body at t = 1 s is velocity × time = (2) × (2) m or 4 m.
Velocity of the body at t = 3 s is 3 m/s.
Displacement of the body at t = 3 s is velocity × time = (3) × (3) m or 9 m
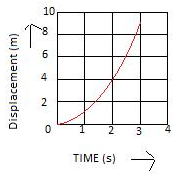
Solution 9N.
(i) Distance travelled in any part of the graph can be determined by finding the area enclosed by the graph in that part with the time axis.
(ii) Distance travelled in part BC = Area of the rectangle tBC2t = base × height.
= (2t – t) × vo
= vot
Distance travelled in part AB = Area of the triangle ABt
= (1/2) × base × height
= (1/2) × t × vo
= (1/2) vo t
Therefore, distance travelled in part BC:distance travelled in part AB :: 2:1.
(iii) (a) BC shows motion with uniform velocity.
(b) AB shows motion with uniform acceleration.
(c) CD shows motion with uniform retardation.
(iv) (a) The magnitude of acceleration is lower as the slope of line AB is less than that of line CD.
(b) Slope of line AB = vo/t
Slope of line CD = vo/0.5t
Slope of line AB/Slope of line CD = (vo /t)/(vo /0.5t)
Slope of line AB:Slope of line CD :: 1:2.
Solution 10N.
(i) Acceleration in the part AB = Slope of AB
= tan (∠BAD)
= (30/4) ms-2
= 7.5 ms-2
Acceleration in the part BC = 0 ms-2
Acceleration in the part CD = slope of CD = -tan (∠CDA)
= -(30/2) ms-2
= -15 ms-2
(ii) Displacement of part AB = Area of ΔAB4 = (1/2) (4) (30)
= 60 m
Displacement of part BC = Area of rectangle 4BC8
= (30) × (4) = 120 m
Displacement of part CD = Area of ΔC8D = (1/2) (2) (30)
= 30 m
(iii) Total displacement = Displacement of part AB + Displacement of part BC + Displacement of part CD
= 60 + 120 + 30 = 210 m
Solution 11N.
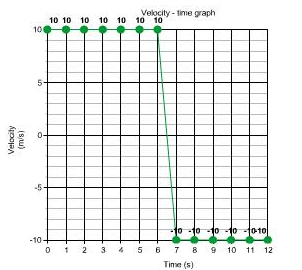
Distance travelled in first 6 s = velocity × time
= 10 m/s × 6
= 60 m/s
Distance travelled in next 6 s = velocity × time
= 10 m/s × 6
= 60 m/s
Total distance travelled in 12 s = (60 + 60) m = 120 m
Total displacement = 0, as the ball returns its starting point.
Solution 12N.
(i) From 0 to 4 seconds, the motion is uniformly accelerated and from 4 to 6 seconds, the motion is uniformly retarded.
(ii) Displacement of the particle at 6 s = (1/2) (6) (2) = 6 m
(iii) The particle does not change its direction of motion.
(iv) Distance travelled by the particle from 0 to 4s (D1) = (1/2) (4) (2) = 4 m
Distance travelled by the particle from 4 to 6s (D2) = (1/2) (2) (2) = 2 m
D1:D2:: 4:2
D1:D2:: 2:1
(v) Acceleration from 0 to 4 s = (2/4) ms-2 or 0.5 ms-2
Retardation from 4 s to 6 s = (2/2) ms-2 or 1 ms-2.
Exercise 2(C)
Solution 1S.
Three equations of a uniformly accelerated motion are
v = u + at
s = ut + (1/2)at2
v2 = u2 + 2as
Solution 2S.
Derivation of equations of motion
First equation of motion:
Consider a particle moving along a straight line with uniform acceleration ‘a’. At t = 0, let the particle be at A and u be its initial velocity, and at t = t, let v be its final velocity.

Acceleration = Change in velocity/Time
a = (v – u)/t
at = v – u
v = u+ at … First equation of motion.
Second equation of motion:
Average velocity = Total distance traveled/Total time taken Average velocity = s/t …(1)
Average velocity can be written as (u+v)/2 Average velocity = (u+v)/2 …(2)
From equations (1) and (2) s/t = (u+v)/2 …(3)
The first equation of motion is v = u + at.
Substituting the value of v in equation (3), we get
s/t = (u + u + at)/2 s = (2u + at) t/2 = 2ut + at2/2 = 2ut/2 + at2/2
s = ut + (1/2) at2 …Second equation of motion.
Third equation of motion:
The first equation of motion is v = u + at. v – u = at … (1)
Average velocity = s/t …(2)
Average velocity =(u+v)/2 …(3)
From equation (2) and equation (3) we get,
(u + v)/2 = s/t …(4)
Multiplying eq (1) and eq (4) we get,
(v – u)(v + u) = at × (2s/t) (v – u)(v + u) = 2as
[We make the use of the identity a2 – b2 = (a + b) (a – b)]
v2 – u2 = 2as …Third equation of motion.
Solution 3S.

Solution 1M.
v = u + at
Solution 2M.
5 km
Solution 1N.
Initial velocity u = 0
Acceleration a = 2 m/s2
Time t = 2 s
Let ‘S’ be the distance covered.
Using the second equation of motion,
S = ut + (1/2) at2
S = 0 + (1/2) (2) (2) 2
S = 4 m
Solution 2N.
Initial velocity u = 10 m/s
Acceleration a = 5 m/s2
Time t = 5s
Let ‘S’ be the distance covered.
Using the second equation of motion,
S = ut + (1/2) at2
S = (10)(5) + (1/2) (5) (5) 2
S = 50 + 62.5
S = 112.5 m
Solution 3N.
Acceleration = Change in velocity/time taken
In the first two seconds,
Acceleration = [(33.6 – 30)/2] km/h2
= 1.8 km/h2
= 0.5 m/s2 …(i)
In the next two seconds,
Acceleration = [(37.2 – 33.6)/2] km/h2
= 1.8 km/h2
= 0.5 m/s2…(ii)
From (i) and (ii), we can say that the acceleration is uniform.
Solution 4N.
Initial velocity u = 0 m/s
Acceleration a = 2 m/s2
Time t = 5 s
(i) Let ‘v’ be the final velocity.
Then, (v – u)/5 = 2
v = 10 m/s
(ii) Let ‘s’ be the distance travelled.
Using the third equation of motion,
v2 – u2 = 2as
We get,
(10) 2 – (0) 2 = 2(2) (s)
Thus, s = (100/4) m = 25 m
Solution 5N.
Initial velocity u = 20 m/s
Final velocity v = 0
Distance travelled s = 10 cm = 0.1 m
Let acceleration be ‘a’.
Using the third equation of motion,
v2 – u2 = 2as
We get,
(0) 2 – (20) 2 = 2(a) (0.1)
a = -(400/0.2) m/s2
a = -2000 m/s2
Thus, retardation = 2000 m/s2
Solution 6N.
Initial velocity u = 20 m/s
Final velocity v = 0
Time taken t = 5 s
Let acceleration be ‘a’.
Using the first equation of motion,
v = u + at
0 = 20 + 5a
a = -4 m/s2
Thus, retardation = 4 m/s2
Solution 7N.
Let ‘s’ be the distance between stations A and B.
(i) Average speed = Total distance/total time taken
Here, total distance = s + s = 2s
Total time taken = Time taken to travel from A to B + Time taken to travel from B to A.
= [(s/ 60) + (s/ 80)] s
= [ 140 s / 4800] s
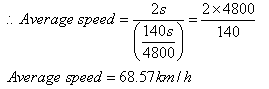
(ii) Average velocity = Displacement/total time taken
Because the train starts and ends at the same station, the displacement is zero. Thus the average velocity is zero.
Solution 8N.
Initial velocity u = 90 km/h = 25 m/s
Final velocity v = 0 m/s
Acceleration a = -0.5 m/s2
(i) Let ‘V’ be the velocity after time t = 10 s
Using the first equation of motion,
v = u + at
We get,
V = 25 + (-0.5) (10) m/s
V = 25 – 5 = 20 m/s
(ii) Let t’ be the time taken by the train to come to rest.
Using the first equation of motion,
v = u + at
We get,
t’ = [(0 – 25)/ (-0.5)] s
t’ = 50 s
Solution 9N.
Distance travelled s = 100 m
Average velocity V = 20 m/s
Final velocity v = 25 m/s
(i) Let u be the initial velocity.
Average velocity = (Initial velocity + Final velocity)/2
V = (u + v)/2
20 = (u + 25)/2
u = 40 – 25 = 15 m/s
(ii) Let ‘a’ be the acceleration of the car.
Using the third equation of motion,
v2 – u2 = 2as
We get,
(25) 2 – (15) 2 = 2 (a) (100)
625 – 225 = 200 a
a = (400/200) m/s2 = 2 m/s2
Solution 10N.
Final velocity v = 0
Acceleration = -25 cm/s2 or -0.25 m/s2
Time taken t = 20 s
(i) Let ‘u’ be the initial velocity.
Using the first equation of motion,
v = u + at
We get,
u = v – at
u = 0 – (-0.25)(20) = 5 m/s
(ii) Let ‘s’ be the distance travelled.
Using the third equation of motion,
v2 – u2 = 2as
We get,
(0) 2 – (5)2 = 2 (-0.25) (s)
s = (25/0.5) = 50 m.
Solution 11N.
Initial velocity u = 0 m/s
Distance travelled s = 270 m
Time taken to travel s distance = 3 s
Let ‘a’ be the uniform acceleration.
Using the second equation of motion,
S = ut + (1/2) at2
We get,
270 = 0 + (1/2) a (3)2
270 = 9a/2
a = 60 m/s2
Let v be the velocity of the body 10 s after the start.
Using the first equation of motion, we get
v = u + at
v = 0 + (60)(10) = 600 m/s
Solution 12N.
Let the constant acceleration with which the body moves be ‘a’.
Given, the body travels distance S1 = 3 m in time t1 = 1 s.
Same body travels distance S2 = 8 m in time t2 = 2 s.
(i) Let ‘u’ be the initial velocity.
Using the second equation of motion,
S = ut + (1/2) at2
Substituting the value for S1 and S2, we get
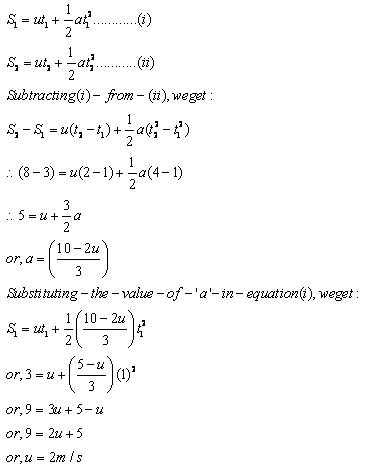
(ii) Putting u = 2 m/s in the equation
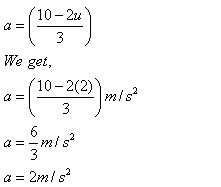
Solution 13N.
Initial velocity u = 25 m/s
Final velocity v = 0
(i) Before the brakes are applied, let S be the distance travelled.
Distance = Speed × time
S = (25) × (5) m
S = 125 m
(ii) Acceleration = (Final velocity – Initial velocity)/Time taken
= [(0 – 25)/15] ms-2
= (-5/2) ms-2
= -2.5 ms-2
Therefore, retardation = 2.5 ms-2
(iii) After applying brakes, the time taken to come to stop = 10 s
Let S’ be the distance travelled after applying the brakes.
Initial velocity u = 25 m/s
Final velocity v = 0
Using the third equation of motion,
v2 – u2 = 2as
We get,
(0) 2 – (25)2 = 2 (-2.5) (S’)
625 = 5(S’)
S’ = 125 m
Solution 14N.
Given, the initial velocity u = 75 km/s
Final velocity v = 120 km/s
Time taken = 6 s
(i) Acceleration = (Final velocity – Initial velocity)/time taken
= [(120 – 75)/6] kms-2
= (45/6) kms-2
= 7.5 kms-2
(ii) Distance travelled by the aircraft in the first 10 s = Distance travelled in the first 6 s + Distance travelled in the next 4 s.
Distance travelled in the first 6s (S1) = ut + (1/2) at2
(S1) = ut + (1/2) at2
(S1) = (75)(6) + (1/2) (7.5)(6)2
(S1) = 450 + 135
(S1) = 585 km
Distance travelled in the next 4 s (S2) = Speed × time
Speed at the end of 6 s is 120 km/s.
(S2) = (120) (4)
(S2) = 480 km
Thus, the distance travelled by the aircraft in the first 10 s = (S1) + (S2) = 585 + 480 = 1065 km.
Solution 15N.
(i) For the first 10 s, initial velocity u = 0
Acceleration a = 2 m/s2
Time taken t = 10 s
Let ‘v’ be the maximum velocity reached.
Using the first equation of motion
v = u + at
We get
V = (0) + (2) (10) = 20 m/s
(ii) For the last 50 s: Final velocity = 0 m/s, initial velocity = 20 m/s.
Acceleration = (Final velocity – Initial velocity)/time
= (0 – 20)/50 = -0.4 m/s2
Retardation = 0.4 m/s2
(iii) Total distance travelled = Distance travelled in the first 10 s + Distance travelled in 200 s + Distance travelled in last 50 s
Distance travelled in first 10s (s1) = ut + (1/2) at2
S1= (0) + (1/2) (2) (10)2
S1= 100 m
Distance travelled in 200s (s2) = speed × time
S2 = (20) (200) = 4000 m
Distance travelled in last 50s (s3) = ut + (1/2) at2
Here, u = 20 m/s, t = 50 s and a = -0.4 m/s2
S3= (20)(50) + (1/2) (-0.4) (50)2
S3= 1000 – 500
S3= 500 m
Therefore, total distance travelled = S1 + S2 + S3 = 100 + 4000 + 500 = 4600 m
(iv) Average velocity = Total distance travelled/total time taken
= (4600/260) m/s
= 17.69 m/s
More Resources for Selina Concise Class 9 ICSE Solutions
![]()