Application of the reactivity series of metals in the extraction of metals
Application of the reactivity series of metals towards oxygen in the extraction of metals:
- Only a few metals such as platinum, gold and silver are found free in nature. Other metals have to be extracted from their ores.
- Ores are naturally occurring rocks that contain high concentration of one or more mineral of metals. The common minerals found in ores are oxides, sulphides and carbonates of metals.
- The following table shows the main contents of some common ores and the metals extracted from them.
Ore Main mineral in ore Metal extracted from ore Carnallite Hydrated potassium magnesium chloride, KCl.MgCl2.6H2O Potassium or magnesium Halite (rock salt) Sodium chloride, NaCl Sodium Bauxite Aluminium oxide, Al2O3 Aluminium Zinc blende (sphalerite) Zinc sulphide, ZnS Zinc Smithsonite Zinc carbonate, ZnCO3 Zinc Hematite Iron(III) oxide, Fe2O3 Iron Magnetite Triiron tetroxide. Fe3O4 Iron Cassiterite Tin(IV) oxide. SnO2 Tin Galena Lead(II) sulphide, PbS Lead Malachite Copper(II) carbonate hydroxide, CuCO3.Cu(OH)2 Copper Chalcopyrite Copper iron sulphide, CuFeS2 Copper Cinnabar Mercury(II) sulphide, HgS Mercury - When a metal is extracted from its ore, it involves reduction.
(a) A metal has a positive oxidation number in its ore.
(b) Hence, when it is extracted from its ore, its oxidation number decreases to zero.
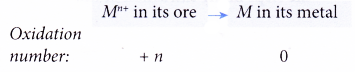
- The method of reduction depends on the reactivity of the metals. More often than not, the cost is also taken into consideration. For example, a metal can be extracted from its ore using more reactive metals as the reducing agents. However, this is usually not done on a large scale as it is expensive.
- Figure summarises the methods of extracting metals from their ores.
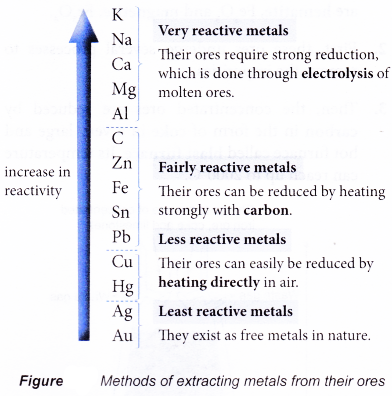
- In extracting metals, carbon in the form of coke is used. Carbon is widely used in the extraction of zinc, iron, tin and lead for a number of reasons:
(a) First of all, carbon is more reactive than zinc, iron, tin and lead. Therefore, carbon can easily reduce the oxide of these metals.
(b) Furthermore, carbon is cheap and easily available.
(c) The carbon dioxide gas produced is not poisonous and thus can be released directly into the air. - Two main processes in the extraction of metals:
(a) Concentrating the ores (this is done by removing the impurities in the ores)
(b) Reducing the ores into metals
People also ask
- The Reactivity Series of Metals Towards Oxygen
- What is a redox reaction?
- Changing of iron(II) ions to iron(III) ions and vice versa
- Redox reaction in the displacement of metals from its salt solution
- Displacement of Halogen From Halide Solution
- Redox Reactions by Transfer of Electrons at a Distance
- Rusting as a Redox Reaction
- Electrolytic and Chemical Cells
- Oxidation and Reduction in Electrolytic Cells
- Oxidation and Reduction in Chemical Cells
- How does a voltaic cell work?
Extraction of iron
- There are a number of iron ores. However, it is uneconomical to extract iron from most of the ores. Two important ores used in extracting iron are hematite, Fe2O3 and magnetite, Fe3O4.
- First, these ores undergo several processes to remove the impurities.
- Then, the concentrated ores are reduced by carbon in the form of coke in a very large and hot furnace called blast furnace. Its temperature can reach up to 2000°C.
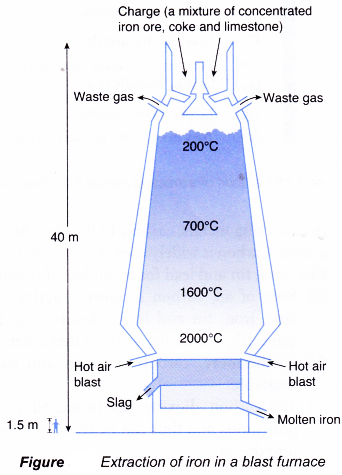
- A small charge is introduced from the top of the blast furnace at intervals of 10 to 15 minutes. The charge consists of concentrated iron ores, coke and limestone.
- The following flow chart outlines the reduction of the iron ores in the blast furnace.
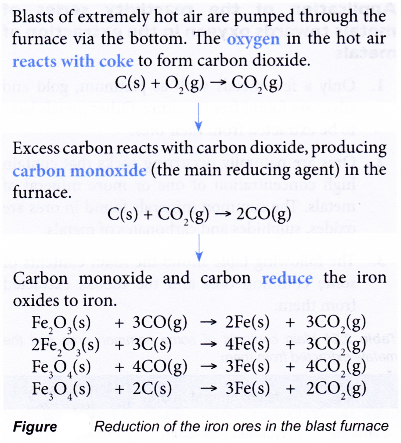
- The molten iron is collected at the bottom of the furnace. It is drained off periodically into moulds and is allowed to cool. The product is called pig iron or cast iron.
- At the same time, the intense heat in the blast furnace causes the limestone to decompose.
CaCO3(s) → CaO(s) + CO2(g)
(a) The calcium oxide then reacts with the impurities in the ores, which consist mostly of sand, SiO2, to form calcium silicate, CaSiO3 or slag.
CaO(s) + SiO2(s) → CaSiO3(l)
(b) As the molten slag is less dense than molten iron, it floats on the molten iron, protecting the molten iron from oxidation by the hot air.
(c) Like the molten iron, the slag is also drained off periodically. The slag can be used as a building material and for the manufacture of cement.
Extraction of tin
- The main ore of tin is cassiterite which contains tin(IV) oxide, SnO2. The following summarises the steps in the extraction of tin.
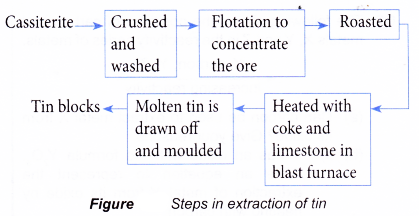
- The ore is first crushed, grounded and washed.
- Then, the ore is concentrated by mixing it with oil and water. In this flotation method, the tin minerals, which are less dense, are trapped in the floating foam. The impurities such as soil and sand, which are denser, sink to the bottom.
- The concentrated ore is then roasted in the air. This converts the sulphide of tin to oxide. At the same time, impurities such as sulphur and oil are burnt off.
- Similar to iron, the reduction of tin(IV) oxide takes place in the blast furnace by carbon monoxide and coke.
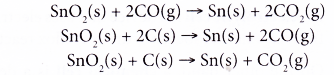
- Calcium oxide from the limestone eliminates the remaining impurities to slag.
- The molten tin is drained off into moulds to become tin blocks.
Contribution of the metal extraction industry
- Malaysia has one of the largest reserves of tin ore in the world and was once the world’s largest producer of tin. Tin played a predominant role in Malaysian economy in the 19th and 20th centuries until the collapse of the tin market in the early 1980s.
- Other than tin ore, significant amount of iron ore, copper ore, bauxite and gold are also mined in our country.
- Besides providing jobs to our people, the local metal extraction industry has contributed greatly to our economy and has supported many local metal-related industries such as the food canning industry, alloy manufacturing and the jewellery industry.
Conserving metals
- Metals are non-renewable and the worlds reserves of metals are depleting. Thus, we need to conserve metals. This can be done through the 3Rs (reduce, reuse and recycle).
- We should reduce the use of metals in whatever way we can. For example, we can use pencil cases made of cloth instead of metal.
- Tin and aluminium cans can be reused as pencil holders. Broken metal furniture can be welded, painted and reused again.
- Two most common recycled metals are aluminium and iron.
- Recycling of metals not only conserves metals, but it also conserves energy and the environment. For example, recycling aluminium uses only 5% of the energy required to produce aluminium from its ore and reduces air pollution by 95%.