Electrolytic and Chemical Cells
- Redox reaction occurs in both electrolytic and chemical cells.
- An electrolytic cell is a device that uses electricity from an external source to drive a redox reaction.
- On the other hand, a chemical cell is a device that uses redox reaction to produce electricity.
- The two cells differ in the following aspects.
| Electrolytic cell | Chemical cell | |
| Basic structure | It requires a source of electric current. The electrodes may be of the same or different materials. | It does not require a source of electric current. The electrodes must be of two different materials. |
| Energy conversion | The supplied electrical energy causes chemical reactions to occur at the electrodes. Electrical energy → chemical energy | The chemical reactions that occur at the electrodes produce an electric current. Chemical energy → electrical energy |
| Tranfer of electrons | Electrons flow from the positive electrode to the negative electrode through the connecting wires. | Electrons flow from the more electropositive metal (negative terminal) to the less electropositive metal (positive terminal). |
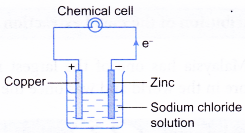 | 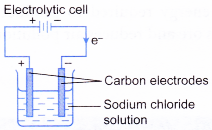 |
- For both electrolytic cell and chemical cell, the terms ‘anode’ and ‘cathode’ are assigned based on the reaction that occurs at the electrodes.
- The electrode at which oxidation occurs is called the anode.
- The electrode at which reduction occurs is called the cathode.
People also ask
- Oxidation and Reduction in Electrolytic Cells
- Oxidation and Reduction in Chemical Cells
- What is a redox reaction?
- Changing of iron(II) ions to iron(III) ions and vice versa
- Redox reaction in the displacement of metals from its salt solution
- Displacement of Halogen From Halide Solution
- Redox Reactions by Transfer of Electrons at a Distance
- Rusting as a Redox Reaction
- The Reactivity Series of Metals Towards Oxygen
- Application of the reactivity series of metals in the extraction of metals
- How does a voltaic cell work?
Types of Chemical Cells
- Chemical cells can be divided into two main types, namely primary cells and secondary cells.
- Primary cells are not rechargeable and can be used only once. They have to be disposed of once they are exhausted or fully discharged.
- Examples of primary cells are dry cells, alkaline cells and mercury cells.
- Secondary cells are rechargeable when they are exhausted and can be used again and again.
- Secondary cells can be recharged by passing an electric current through them in the opposite direction to the current flow during discharge or use.
- Examples of secondary cells include lead-acid accumulator, nickel-cadmium cell and lithium- ion cells.
Dry cell
Dry cell is a primary cell and is available in various sizes, from the big battery in a flashlight to the small battery in a remote control. However, its main structure remains the same:
(a) Anode(-): Zinc casing
(b) Cathode(+): Carbon rod
(c) Electrolyte: Moist paste of ammonium chloride
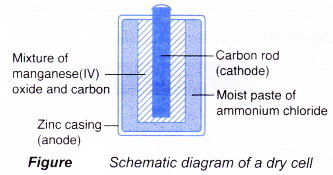
The reactions that occur during the use of a dry cell are as follows.
- At the anode, zinc releases electrons and is oxidised to zinc ions. Thus, zinc acts as the reducing agent.
- The electrons flow through the external circuit to the cathode.
- At the cathode, ammonium ions act as the oxidising agent, accepting the electrons, and thus are reduced to hydrogen gas. The oxidation number of hydrogen decreases from +1 in ammonium ions to 0 in hydrogen gas.
- Thus, the overall reaction is as follows.
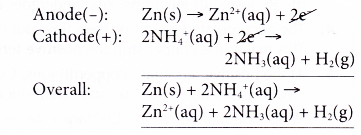
The hydrogen gas produced is eliminated by manganese(IV) oxide.
Alkaline cell
Alkaline cells are primary cells with a longer shelf life than dry cells.
Main structure of an alkaline cell:
(a) Anode(-): Zinc powder
(b) Cathode(+): Manganese(IV) oxide (carbon is added to it to increase its electrical conductivity)
(c) Electrolyte: Potassium hydroxide paste
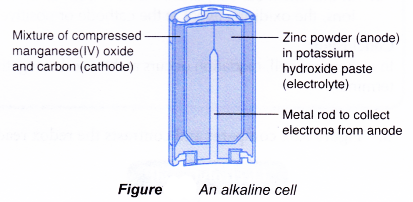
The reactions that occur during -the use of an alkaline cell are as follows.
- At the anode, zinc powder acts as the reducing agent, losing electrons and is oxidised to zinc ions.
- The electrons collected at the metal rod flow through external circuit to the cathode.
- At the cathode, manganese(IV) ions, the oxidising agent, accept the electrons and are reduced to manganese(III) ions. The oxidation number of manganese decreases from +4 in manganese(IV) ions to +3 in manganese(III) ions.
- Hence, the overall reaction is as follows.
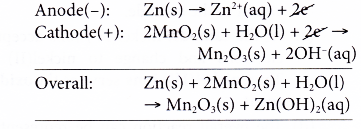
Mercury cell
Very small button-shaped cells are needed for devices such as watches and cameras. One of the common button-shaped cells is mercury cell.
Main structure of a mercury cell:
(a) Anode(-): Zinc powder
(b) Cathode(+): Mercury(II) oxide (carbon is added to it to increase its electrical conductivity)
(c) Electrolyte: Potassium hydroxide paste
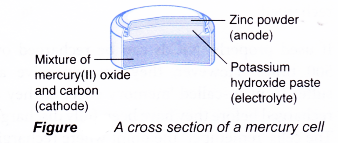
The reactions that occur during the use of a mercury cell are as follows.
- At the anode, zinc powder releases electrons and is oxidised to zinc ions. Thus, zinc acts as the reducing agent.
- The electrons flow through external circuit to the cathode.
- At the cathode, mercury(II) ions gain the electrons and are reduced to metallic mercury. Thus, mercury(II) ions are the oxidising agent.
- The overall reaction is as follows.
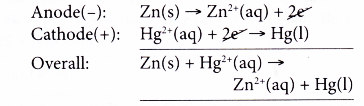
The metallic mercury produced poses a danger to the environment. Hence, used mercury cells should be recycled. After collection, the cells are broken up and the mercury is recycled.
Lead-acid accumulator
Lead-acid accumulator is rechargeable and is well known for its use as car battery.
Each cell has the following main structure:
(a) Anode(-): Metallic lead.
(b) Cathode(+): Lead(IV) oxide
(c) Electrolyte: Sulphuric acid
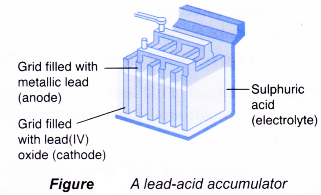
The reactions that occur during the discharge or use of a lead-acid accumulator are as follows.
- At the anode, metallic lead acts as the reducing agent, releasing electrons to form lead(II) ions. This is an oxidation process.
- The electrons released travel through the external circuit to run lights, starters or air- conditioner. The electrons then travel to the cathode to complete the circuit.
- At the cathode, lead(IV) ions gain the electrons and are reduced to lead(II) ions. Thus, lead(IV) ions act as the oxidising agent.
- Lead(II) ions formed at both anode and cathode combine with sulphate ions in the electrolyte to form insoluble lead(II) sulphate.
- Hence, the overall reaction can be represented as follows.
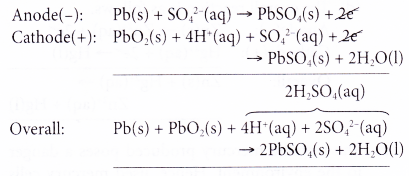
As the redox reaction proceeds, both electrodes turn to lead(II) sulphate and the sulphuric acid turns to water. Thus, the accumulator slowly loses its power and eventually becomes ‘dead’.
When this happens, the accumulator can be recharged by passing an electric current in the opposite direction. This causes the reverse reaction to take place.
![]()
Improper disposal of old lead-acid accumulators could lead to environmental hazard such as lead-poisoning.
In the recycling of lead-acid accumulators, the accumulators are broken, the electrolyte is neutralised and the lead is recovered through controlled processes. The lead is then refined for resale.
Nickel-cadmium cell
- Nickel-cadmium cell, or commonly abbreviated as NiCd or NiCad, is a popular rechargeable battery for toys and electronic devices such as remote controls. They are available in various sizes and voltages.
- Today, almost all NiCds use the ‘jelly-roll’ design whereby several layers of anode and cathode materials are rolled into a cylindrical shape.
- Each cell has the following main structure:
(a) Anode (-): Cadmium
(b) Cathode(+): Nickel(IV) oxide
(c) Electrolyte: Potassium hydroxide
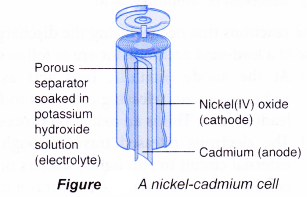
- The reactions that occur during the discharge or use of a nickel-cadmium cell are as follows.
(a) At the anode, cadmium releases electrons and thus becomes the reducing agent.
(b) The electrons travel through the external circuit to the cathode.
(c) At the cathode, nickel(IV) ions accept the electrons and change to nickel(II) ions. Thus, nickel(IV) ions serve as the oxidising agent.
(d) The overall reaction can be represented as follows.
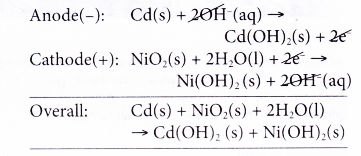
- The overall reaction is reversed when the cell is recharged.
- If used properly, NiCds can be recharged over 500 times. However, they are expensive and suffer from so-called ‘memory effect’ if they are recharged before they have been fully discharged. The cells ‘remember’ the point where recharging began and during subsequent use, they suffer a sudden drop in voltage at that point.
- NiCds contain cadmium, which is a toxic heavy metal. Therefore, they should be disposed of properly. The heavy metal can be recovered through recycling.
Other chemical cells
There are several new rechargeable chemical cells available in the market. These include the nickel-metal hydride, lithium-ion and lithium polymer cells.
Nickel-metal hydride (NiMH)
- NiMHs are similar to NiCds but they do not contain cadmium. Instead, they contain compounds of rare earth elements such as titanium, vanadium, zirconium, cobalt, manganese and aluminium. Thus, these cells are cheaper and considered more environmentally friendly.
- NiMHs have higher capacity than’NiCds. Thus, they are usually used in high drain devices such as digital cameras and mobile phones.
- They are less prone to memory effect but have a higher self-discharge rate than NiCds.
Lithium-ion (Li-Ion)
- Currently, Li-Ion is the most popular rechargeable battery for mobile phones and notebook computers.
- This is because Li-Ion is smaller and therefore lighter. Furthermore, it does not suffer memory effect like NiCds.
- A Li-Ion has carbon as the anode and a metal oxide as the cathode. It has a special solid-state electrolyte, which is a lithium salt in an organic solvent-such as ether. The metallic lithium produced during use is very reactive and might cause explosion. Thus, a Li-Ion is inflammable and can easily explode when exposed to high temperature.
- The shelf life of a Li-Ion is dependent upon aging from the time of manufacture, regardless of whether it is used or not.
Lithium-polymer (Li-Poly)
- Li-Poly is a more advanced design of Li-Ion.
- The difference is that the lithium salt electrolyte is not held in an organic solvent but is held in a solid polymer composite such as polyacrylonitrile. Hence, Li-Poly is not flammable.
- Another advantage of Li-Poly is that it can be made very thin, small and light, a feature which is highly desired in portable electronic devices such as mobile phones, MP3 players and Bluetooth enabled devices.
- Generally, almost all resources on earth are depleting. Hence, scientists are looking into possibilities of developing chemical cells such as fuel cells and solar cells as alternative sources of renewable energy.
Fuel cells
- A fuel cell converts the chemical energy of a fuel such as hydrogen and natural gas to electricity.
- A fuel cell consists of an anode (to which a fuel is supplied) and a cathode (to which air or oxygen is supplied) which are separated by an electrolyte.
- A hydrogen fuel cell runs on hydrogen which is obtainable from the electrolysis of water. Hydrogen is constantly fed to the anode while oxygen is constantly fed to the cathode.
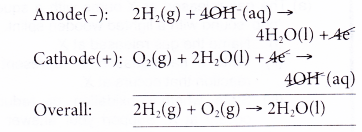
In this example, the only waste product is water, a non-polluting product. - Scientists are trying to make fuel cells economically competitive. At the moment, fuel cells are only used as an energy source in remote locations, such as in spacecraft, remote weather stations or in certain military applications.
Solar cells
- Solar cells are made from thin slices of semiconductor materials such as crystalline silicon or gallium arsenide.
- These cells are attractive as they can convert solar energy, a renewable source of energy, directly into electricity. Furthermore, they are pollution-free.
- Due to high cost, solar cells are currently used to power orbiting space satellites, gates at unattended railroad crossings and irrigation pumps.
Follow us on:
https://plus.google.com/+Veerendracbse
https://plus.google.com/+DurgaprasannaAmujuri
https://plus.google.com/+phanicbse
https://plus.google.com/+vinilachoudary
https://plus.google.com/+LearnCbseind
https://plus.google.com/+kajalsharmaLearnCBSE
https://plus.google.com/+sravyasharma
https://plus.google.com/+dattuvalipishetty
https://plus.google.com/+kajalsharmaLearnCBSE
https://plus.google.com/+cbseguesspapersfree
https://plus.google.com/+CharanSing
https://plus.google.com/+rajuheal
https://plus.google.com/+saralasharma
https://plus.google.com/+mastiaddaLearnCBSE
https://plus.google.com/+ChinnaDarling
https://plus.google.com/+ChandraRamLearnCBSE
https://plus.google.com/+AmrutPandey
https://plus.google.com/+Rajashekharvalipishetty
https://plus.google.com/+VenkataLakshmiLearnCBSE
https://plus.google.com/+nagaRajuLearnCBSE
https://plus.google.com/+SekharThe
https://plus.google.com/+TriveniMcc