Changing of iron(II) ions to iron(III) ions and vice versa
- Iron exhibits two oxidation numbers
(a) +2 as iron(II) ion, Fe2+
(b) +3 as iron(III) ion, Fe3+ - An aqueous solution containing iron(II) ions, Fe2+ is pale green in colour, whereas that containing iron(III) ions, Fe3+ is yellow/yellowish-brown/ brown in colour.
- Changing iron(II) ions to iron(III) ions is an oxidation and therefore requires an oxidising agent.
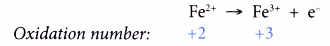
- On the other hand, changing iron(III) ions to iron(II) ions is a reduction and therefore requires a reducing agent.
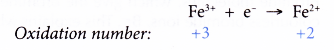
Table: Detecting the presence of iron(II) ions and iron(III) ions
| Reagent | With iron ions | Observation |
| Sodium hydroxide solution or ammonia solution | Fe2+ | Green precipitate, insoluble in excess alkali |
| Fe3+ | Brown precipitate, insoluble in excess alkali | |
| Potassium hexacyanoferrate(ll) solution | Fe2+ | Light blue precipitation |
| Fe3+ | Dark blue precipitation | |
| Potassium hexacyanoferrate(lll) solution | Fe2+ | Dark blue precipitation |
| Fe3+ | Greenish-brown solution | |
| Potassium/ammonium thiocyanate solution | Fe2+ | Pale red colouration |
| Fe3+ | Blood-red colouration |
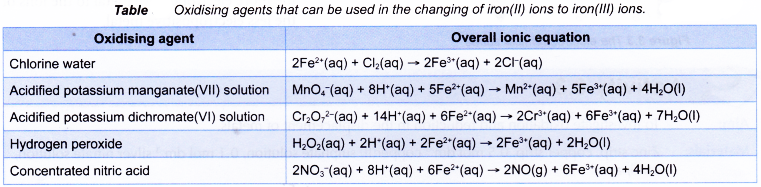
The following are other oxidising agents that can replace bromine water in changing iron(II) ions to iron(III) ions.
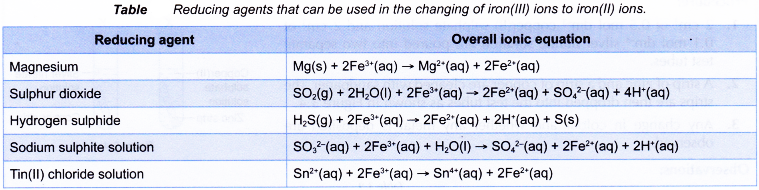
Other reducing agents that can replace zinc powder in changing iron(III) ions to iron(II) ions are as follows.
People also ask
- What is a redox reaction?
- Redox reaction in the displacement of metals from its salt solution
- Displacement of Halogen From Halide Solution
- Redox Reactions by Transfer of Electrons at a Distance
- Rusting as a Redox Reaction
- The Reactivity Series of Metals Towards Oxygen
- Application of the reactivity series of metals in the extraction of metals
- Electrolytic and Chemical Cells
- Oxidation and Reduction in Electrolytic Cells
- Oxidation and Reduction in Chemical Cells
- How does a voltaic cell work?
Changing of iron(II) ions to iron(III) ions and vice versa experiment
Aim: To investigate oxidation and reduction in the change of iron(II) ions to iron(III) ions and vice versa.
Materials: 0.5 mol dm-3 freshly prepared iron(II) sulphate solution, 0.5 mol dm-3 iron(III) sulphate solution, bromine water, zinc powder, 2.0 mol dm-3 sodium hydroxide solution, filter paper.
Apparatus: Dropper, spatula, test tubes, test tube holder, Bunsen burner, filter funnel, test tube rack.
Procedure:
A. Changing of iron(II) ions to iron(III) ions
- 2 cm3 of 0.5 mol dm-3 iron(II) sulphate solution is poured into a test tube.
- Using a dropper, bromine water is added to the solution drop by drop.
- The test tube is warmed gently.
- 2.0 mol dm-3 sodium hydroxide solution is added slowly to the mixture until in excess.
B. Changing of iron(III) ions to iron(II) ions
- 2 cm3 of 0.5 mol dm-3 iron(III) sulphate solution is poured into a test tube.
- Half a spatula of zinc powder is added to the solution.
- The mixture is filtered.
- 2.0 mol dm-3 sodium hydroxide solution is added slowly to the filtrate until in excess.
Observations:
| Activity | Reagent | Observations |
| A | Bromine water | Bromine water decolourises. The solution changes colour from pale green to yellow. |
| Sodium hydroxide solution | Brown precipitate is formed. It is insoluble in excess alkali. | |
| B | Zinc powder | Some of the zinc powder dissolves. The solution changes colour from brown to pale green. |
| Sodium hydroxide solution | Green precipitate is formed. It is insoluble in excess alkali. |
Discussion:
A. Changing of iron(II) ions to iron(III) ions
- Bromine water oxidises iron(II) ions, Fe2+ to iron(III) ions, Fe3+. The presence of Fe3+ ions is confirmed by the formation of brown precipitate with sodium hydroxide solution.
- Fe2+ ions lose their electrons and are oxidised to Fe3+ ions.
- Bromine molecules, which give the bromine water its brown colour, gain the electrons and are reduced to colourless bromide ions, Br–. This explains why the bromine water is decolourised.
- In this reaction, bromine water acts as the oxidising agent, where as Fe2+ ions act as the reducing agent.
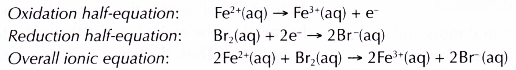
B. Changing of iron(III) ions to iron(II) ions
- Zinc powder reduces iron(III) ions, Fe3+ to iron(II) ions, Fe2+. The presence of Fe2+ ions is confirmed by the formation of green precipitate with sodium hydroxide solution.
- Zinc atoms lose their electrons and are oxidised to zinc ions, Zn2+. This explains why zinc powder dissolves in iron(III) sulphate solution.
- Fe3+ ions accept these electrons and are reduced to Fe2+ ions.
- In this reaction, Fe3+ ions act as the oxidising agent, whereas zinc acts as the reducing agent.
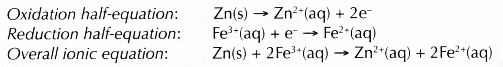
Conclusion:
- Bromine water acts as an oxidising agent, changing iron(II) ions to iron(III) ions.
- Zinc acts as a reducing agent, changing iron(III) ions to iron(II) ions.