What is a redox reaction?
- Many processes that occur around us are redox reactions. These include combustion, rusting, photosynthesis, respiration and decomposition.
- Redox reactions are chemical reactions involving oxidation and reduction occurring simultaneously.
- Therefore, redox reaction is also known as oxidation-reduction reaction.
- It is interesting to note that oxidation is always accompanied by reduction. Both oxidation and reduction have to occur simultaneously.
- Redox reactions can be explained based on:
(a) Loss or gain of oxygen
(b) Loss or gain of hydrogen
(c) Transfer of electrons
(d) Changes in oxidation number
Types of redox reactions
- Not all chemical reactions are redox reactions. For example, acid-base reactions and double decomposition reactions (as in the precipitation method) are non-redox reactions.
- Four examples of redox reactions are as follows:
(a) Changing of iron(II) ions to iron(III) ions and vice versa
(b) Displacement of metal from its salt solution
(c) Displacement of halogen from its halide solution
(d) Transfer of electrons at a distance
People also ask
- Changing of iron(II) ions to iron(III) ions and vice versa
- Redox reaction in the displacement of metals from its salt solution
- Displacement of Halogen From Halide Solution
- Redox Reactions by Transfer of Electrons at a Distance
- Rusting as a Redox Reaction
- The Reactivity Series of Metals Towards Oxygen
- Application of the reactivity series of metals in the extraction of metals
- Electrolytic and Chemical Cells
- Oxidation and Reduction in Electrolytic Cells
- Oxidation and Reduction in Chemical Cells
- How does a voltaic cell work?
What is oxidation and reduction reaction?
Redox reactions based on loss or gain of oxygen
- Originally, chemists explain redox reactions in terms of loss or gain of oxygen.
- Oxidation is a gain of oxygen.
- Therefore, when a substance gains oxygen, it is said to be oxidised.
- The substance that causes oxidation is called the oxidising agent or oxidant.
- Reduction is the opposite of oxidation. Reduction is a loss of oxygen.
- Therefore, when a substance loses its oxygen, it is said to be reduced.
- The substance that causes reduction is called the reducing agent or reductant.
- The following is another redox reaction involving oxygen.

(a) Zinc undergoes oxidation because it gains oxygen to form zinc oxide.
(b) Lead(II) oxide causes zinc to be oxidised. So, lead(II) oxide is the oxidising agent in this redox reaction.

(c) At the same time, lead(II) oxide undergoes reduction because it loses its oxygen to zinc. It is reduced to metallic lead.
(d) Zinc causes lead(II) oxide to be reduced. So, zinc acts as the reducing agent.
Redox reactions based on loss or gain of hydrogen
- Not all redox reactions involve oxygen. For redox reactions involving hydrogen gas or substances containing hydrogen, it is easier to explain the oxidation and reduction in terms of loss or gain of hydrogen.
- Oxidation is a loss of hydrogen, whereas reduction is a gain of hydrogen.
- Therefore, when a substance loses its hydrogen, it is said to be oxidised.
- Take the following reaction as an example.
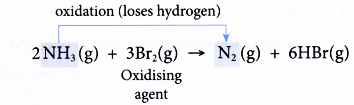
(a) Ammonia is oxidised as it loses its hydrogen to form nitrogen gas.
(b) Bromine is the oxidising agent as it causes ammonia to be oxidised.
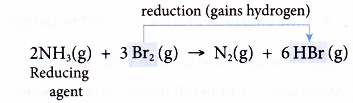
(c) At the same time, bromine is reduced as it gains the hydrogen lost by ammonia to form hydrogen bromide.
(d) Ammonia is the reducing agent as it causes bromine to be reduced. - Consider the reaction between hydrogen sulphide and chlorine to produce sulphur and hydrogen chloride.

(a) Hydrogen sulphide is oxidised to sulphur as it loses its hydrogen to chlorine.
(b) Chlorine gains the hydrogen and therefore is reduced to hydrogen chloride.
(c) Chlorine acts as the oxidising agent because it causes hydrogen sulphide to be oxidised.
(d) Hydrogen sulphide acts as the reducing agent because it causes chlorine to be reduced.
Redox reactions based on transfer of electrons
- Many redox reactions do not involve oxygen or hydrogen. These reactions can be explained based on the transfer of electrons that occurred.
- Oxidation is a loss of electrons and reduction is a gain of electrons.
- Thus, the electron acceptor acts as the oxidising agent and the electron donor acts as the reducing agent.
- Consider the following reaction.

(a) In this reaction, there is a transfer of electrons from sodium to chlorine.
(b) This reaction can be taken as two separate changes occurring at the same time. Each change is called a half-reaction and its equation is called a half-equation.
Oxidation half-equation:
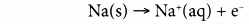
Each sodium atom is oxidised as it loses one electron to form a sodium ion.
Reduction half-equation:
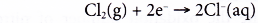
Each molecule of chlorine is reduced as it accepts two electrons from sodium atoms to form two chloride ions.
(c) Sodium is the electron donor and therefore is the reducing agent. On the other hand, chlorine is the electron acceptor. Thus, chlorine acts as the oxidising agent.
(d) We can get the overall equation by adding up the two half-equations.
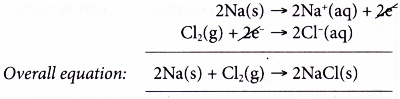
- Zn(s) + CuSO4(aq) → ZnSO4(aq) + Cu(s)
The two half-reactions can be represented as follows.
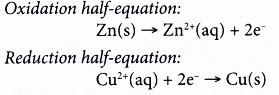
(a) Zinc undergoes oxidation as it loses electrons to form zinc ions.
(b) The electrons are accepted by copper(II) ions to form metallic copper.
(c) Zinc acts as the reducing agent, whereas copper(II) ions act as the oxidising agent.
(d) The overall equation is given below.
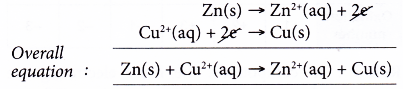
Note: This overall equation is also an ionic equation (an equation without the spectator ions).
Redox reactions based on changes in oxidation number
- Complete transfer of electrons occurs during redox reactions involving ions. There are, however, redox reactions involving molecules that do not involve complete transfer of electrons.
- Redox reactions such as these can be explained in terms of changes in oxidation number.
- Oxidation number or oxidation state of an element is the charge that the atom of the element would have if complete transfer of electrons takes place.
- Each element in a substance can be assigned with an oxidation number. Chemists assign the numbers according to a set of rules.
Rules for assigning oxidation number
Rule 1 : The oxidation number of an atom in its elemental state is zero. For example, C, Na, Mg, Al, H2, O2, Cl2 and Br2 have the oxidation number of 0.
Rule 2 : The oxidation number of a monoatomic ion is equal to its charge.

Rule 3 : In compounds, the more electronegative elements are given a negative oxidation number. The sequence of electronegativity of some elements is shown below.

Therefore, the following rules are adopted.
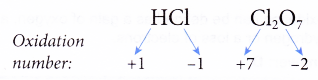
(a) The oxidation number of fluorine in all its compounds is -1 as it is very electronegative.
(b) The oxidation numbers of other halogens (chlorine, bromine and iodine) in their compounds are -1 except when they combine with more electronegative elements such as oxygen and nitrogen.
For example,
(c) The oxidation number of hydrogen in a compound is always +1 except when hydrogen combines with reactive metals in metal hydrides, where it is -1.
For example,
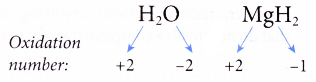
(d) The oxidation number of oxygen in a compound is always -2 except in peroxides and when oxygen combines with a more electronegative element such as fluorine.
For example,
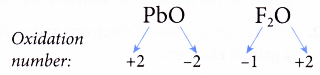
Rule 4 : The sum of the oxidation numbers of all the elements in the formula of a compound must be zero.
For example,
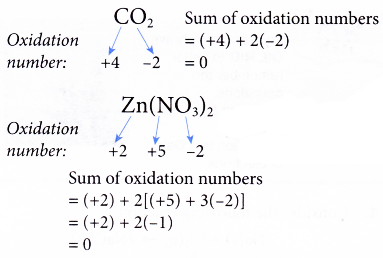
Rule 5 : The sum of the oxidation numbers of all the elements in the formula of a polyatomic ion must be equal to the charge of the ion.
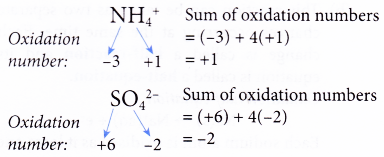
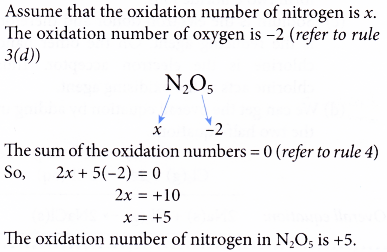
Oxidation Number Example 1. Determine the oxidation number of nitrogen in N2O5.
Solution:
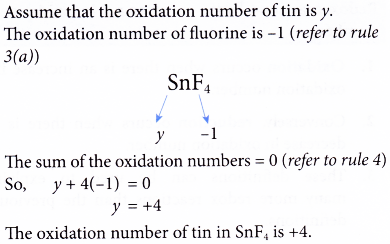
Oxidation Number Example 2. Determine the oxidation number of tin in SnF4.
Solution:
Oxidation Number Example 3. Determine whether each of these reactions is a redox reaction or a non-redox reaction.
I: 2KClO3(s) → 2KCl(s) + 3O2(g)
II: CaO(s) + 2HCl(aq) → CaCl2(aq) + H20(l)
Solution:
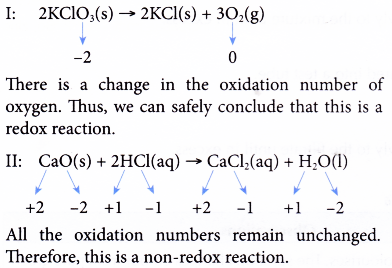
Oxidation number and nomenclature of compounds
- Many elements, especially transition metals, exhibit more than one oxidation number in their compounds. To avoid confusion, oxidation numbers are included in the nomenclature or naming of their compounds.
- For example, oxidation numbers are included in names of simple ionic compounds in the IUPAC nomenclature. The oxidation number of a metal ion is represented by a Roman numeral in brackets, immediately following the name of the metal. Table illustrates some examples.
Formula Oxidation number of metal ion IUPAC name FeO Iron(II) oxide +2 Fe2O3 Iron(III) oxide +3 CuCl Copper(I) chloride +1 CuCl2 Copper(II) chloride +2 PbO Lead(II) oxide +2 PbO2 Lead(IV) oxide +4 MnO Manganese(II) oxide +2 Mn2O3 Manganese(III) oxide +3 MnO2 Manganese(IV) oxide +4 - For elements that have only one oxidation number such as those in Groups 1, 2 and 13, their oxidation numbers are not included in their names.
- Oxidation numbers are also included in the systematic naming of anions containing metals that, can take more than one oxidation number. The oxidation number is represented by a Roman numeral in brackets, immediately following the name of the anion.
Formula Systematic name Traditional name KMnO4 Potassium manganate(VII) Potassium permanganate K2CrO4 Potassium chromate(VI) Potassium chromate K2Cr2O7 Potassium dichromate(VI) Potassium dichromate - For non-metal elements that exhibit more than one oxidation number, the oxidation numbers are written as Roman numerals in brackets, immediately following the name of ions containing them.
Formula Systematic name Traditional name KNO2 Potassium nitrate(III) Potassium nitrite KNO3 Potassium nitrate(V) Potassium nitrate HNO2 Nitric(III) acid Nitrous acid HNO3 Nitric(V) acid Nitric acid H2SO3 Sulphuric(IV) acid Sulphurous acid H2SO4 Sulphuric(VI) acid Sulphuric acid NaOCl Sodium chlorate(I) Sodium hypochlorite NaClO3 Sodium chlorate(V) Sodium chlorate - Some of the systematic names shown in Tables were originally introduced by the Stock system. The traditional names, however, are adopted in the IUPAC nomenclature as they already existed for a long time and they are much easier to use.
Redox reactions based on changes in oxidation number
- Oxidation occurs when there is an increase in oxidation number.
- Conversely, reduction occurs when there is a decrease in oxidation number.
- These definitions can be used to explain many more redox reactions than the previous definitions.
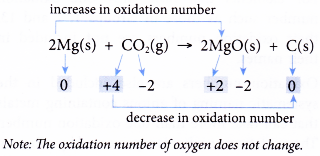
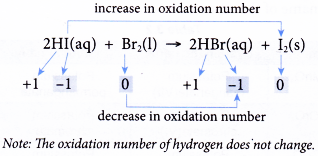
- Table shows how a few redox reactions are explained in terms of changes in oxidation number. First, each element in all substances is assigned with an oxidation number. Then, the changes in the oxidation numbers are analysed.
| Reaction | Explanation |
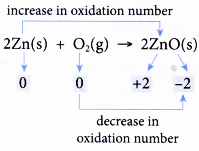 |
|
 |
|
 |
|
- In non-redox reactions, the oxidation numbers of all elements remain unchanged.
- Determining whether a reaction is a redox reaction or a non-redox reaction:
(a) If one of the elements shows a change in oxidation number, it is sufficient to conclude that the reaction is a redox reaction. You need not work out the oxidation numbers of the other elements. This is because an increase in oxidation number is always accompanied by a decrease in oxidation number.
(b) The oxidation numbers of elements in polyatomic ions such as NH4+, SO42- and NO3– need not be determined if the ions appear both in the reactants and in the products.
Redox Reaction Experiment Discussion
Aim: To investigate redox reaction involving oxygen.
A. Combustion of metal in oxygen
Materials: Magnesium ribbon, sandpaper, gas jar containing oxygen gas.
Apparatus: Tongs, Bunsen burner.
Procedure:
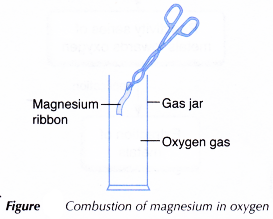
- A piece of 5 cm magnesium ribbon is cleaned with sandpaper.
- Using a pair of tongs, the ribbon is lit and quickly placed into a gas jar filled with oxygen gas. Observation is made.
Observations:
The magnesium ribbon burns with a bright white flame producing a white ash.
Discussion:
- Magnesium burns in oxygen to produce the white ash of magnesium oxide.
- The equation representing the combustion of magnesium:
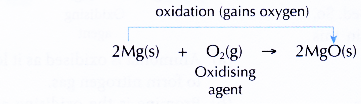
- Magnesium gains oxygen to form magnesium oxide. Therefore, magnesium is said to be oxidised to magnesium oxide.
- The oxidation process is caused by oxygen gas. Thus, oxygen gas is said to act as the oxidising agent (oxidant).
B. Heating of metal oxide with carbon
Materials: Copper(II) oxide, carbon powder.
Apparatus: Crucible, pipe-clay triangle, tripod stand, spatula, Bunsen burner.
Procedure:
- A spatulaful of copper(II) oxide and a spatulaful of carbon powder are mixed thoroughly in a crucible.
- The apparatus is set up as shown in Figure.
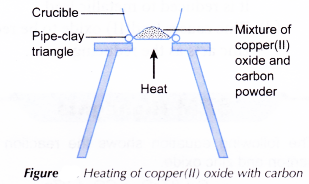
- The mixture is heated strongly. Observation is made.
Observations:
- The mixture burns brightly.
- Reddish-brown globules are formed.
Discussion:
- When copper(II) oxide is heated with carbon, it produces copper which is reddish-brown in colour.
- The equation representing the reaction that occurs:

- Copper(II) oxide loses its oxygen to form copper. Thus, copper(II) oxide is said to be reduced to copper.
- The reduction is caused by carbon. Therefore, carbon is said to act as the reducing agent (reductant).
- Simultaneously, carbon gains oxygen to form carbon dioxide. Carbon is oxidised to carbon dioxide.
- The oxidation of carbon is brought about by copper(II) oxide. So, copper(II) oxide acts as the oxidising agent (oxidant).
Conclusion:
- In the combustion of metal in oxygen, the metal is oxidised by oxygen to metal oxide.
- In the heating of metal oxide with carbon, the metal oxide is reduced by carbon to metal. Simultaneously, carbon is oxidised by the metal oxide to carbon dioxide.