Oxidation and Reduction in Electrolytic Cells
- In an electrolytic cell, an electric current is passed through an electrolyte using electrodes.
- The electrolyte may be a molten ionic compound or an aqueous solution containing ions.
- The electrodes are usually inert conductors such as platinum or carbon. Sometimes active electrodes such as copper are used.
- During electrolysis, the electrolyte undergoes chemical changes at the electrodes. The chemical change at each electrode is actually a half-reaction of a redox reaction.
- The following table shows the oxidation and reduction in a few other electrolytic cells.
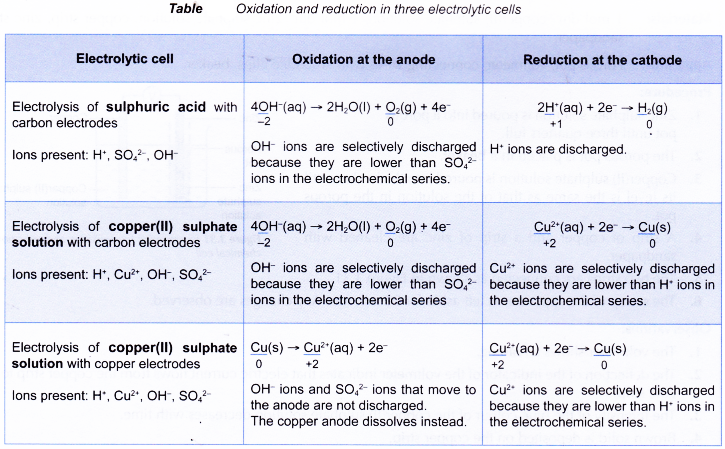
- In all electrolytic cells, electrons flow from the reducing agent at the anode to the oxidising agent at the cathode.
- The reducing agent loses electrons and undergoes oxidation at the anode.
- On the other hand, the oxidising agent gains electrons and undergoes reduction at the cathode.
People also ask
- Electrolytic and Chemical Cells
- Oxidation and Reduction in Chemical Cells
- What is a redox reaction?
- Changing of iron(II) ions to iron(III) ions and vice versa
- Redox reaction in the displacement of metals from its salt solution
- Displacement of Halogen From Halide Solution
- Redox Reactions by Transfer of Electrons at a Distance
- Rusting as a Redox Reaction
- The Reactivity Series of Metals Towards Oxygen
- Application of the reactivity series of metals in the extraction of metals
- How does a voltaic cell work?
Oxidation and Reduction in Electrolytic Cells Experiment
Aim: To investigate the oxidation and reduction in electrolytic cells.
Materials: Solid lead(II) bromide, 1 mol dm-3 potassium iodide solution, 1% starch solution, sandpaper, wooden
Apparatus: Crucible, cardboard, battery, connecting wires with crocodile clips, tripod stand, Bunsen burner, pipe-clay triangle, electrolytic cell, carbon electrodes, switch, ammeter, small test tubes, beaker, tongs.
Procedure:
A. Electrolytic cell involving molten electrolyte
- A crucible is half-filled with solid lead(II) bromide.
- The apparatus is set up as shown in Figure.
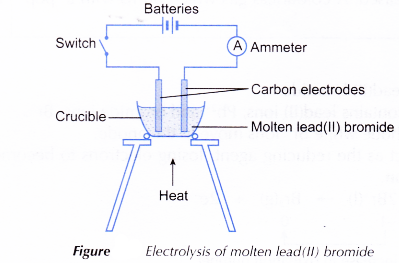
- The solid lead(II) bromide is heated until it is completely melted.
- The switch is turned on to allow electricity to pass through the molten lead(II) bromide for about 20 minutes. Any changes are observed.
- After 20 minutes, the switch is turned off and both electrodes are taken out from the electrolyte. The molten electrolyte is carefully poured into a beaker using tongs. The product left at the bottom of the crucible is observed.
B. Electrolytic cell involving aqueous electrolyte
- An electrolytic cell is half-filled with 0.5 mol dm-3 potassium iodide solution.
- The apparatus is set up as shown in Figure.
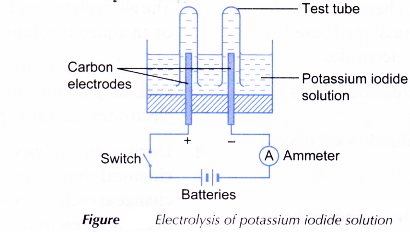
- The switch is turned on to allow electricity to pass through the electrolyte for 15 minutes. Any changes at the anode and cathode are observed.
- The product at the anode is tested with 1 % starch solution while the gas collected at the cathode is tested with a lighted wooden splinter.
Results:
A. Electrolytic cell involving molten electrolyte
| Electrode | Observation | Inference |
| Anode | A brown gas with a pungent and choking smell is released. | Bromine gas is released. |
| Cathode | A shiny grey globule is found at the bottom of the crucible. | Lead is produced. |
B. Electrolytic cell involving aqueous electrolyte
| Electrode | Observation | Inference |
| Anode | The solution in the test tube turns from colourless to brown. It gives a dark blue colouration when tested with starch solution. | Iodine is produced. |
| Cathode | Gas bubbles are released. A colourless gas which burns with a ‘pop’ sound is produced. | Hydrogen gas is produced. |
Discussion:
- In the electrolysis of molten lead(II) bromide:
(a) Molten lead(II) bromide contains lead(II) ions, Pb2+ and bromide ions, Br–.
(b) Pb2+ ions move to the cathode while Br– ions move to the anode.
(c) At the anode, Br– ions act as the reducing agent, losing electrons to become bromine molecules. Thus, Br– ions undergo oxidation.
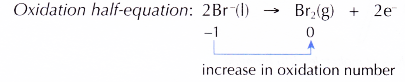
At the cathode, Pb2+ ions act as the oxidising agent, accepting electrons to become metallic lead. Thus, Pb2+ ions undergo reduction.
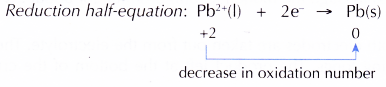
(d) Hence, electrons are transferred from Br– ions, the reducing agent, at the anode to Pb2+ ions, the oxidising agent, at the cathode.
(e) The overall equation is as follows.
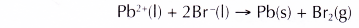
- In the electrolysis of potassium iodide solution:
(a) Potassium iodide solution contains hydrogen ions, H+, potassium ions, K+, hydroxide ions, OH– and iodide ions, I–.
(b) H+ ions and K+ ions move to the cathode while OH– ions and I– ions move to the anode.
(c) At the anode, I– ions are selectively discharged because of their high concentration in the electrolyte. I– ions act as the reducing agent, losing electrons to become iodine molecules. In other words, I ions undergo oxidation.
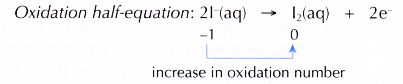
(d) At the cathode, H+ ions are selectively discharged because their position in the electrochemical series is lower than K+ ions. H+ ions act as the oxidising agent, gaining electrons to become hydrogen molecules. In other words, H+ ions undergo reduction.

(e) The overall equation is as follows.
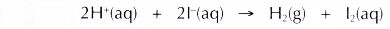
Conclusion:
In an electrolytic cell, oxidation occurs at the anode (positive electrode) while reduction occurs at the cathode (negative electrode).