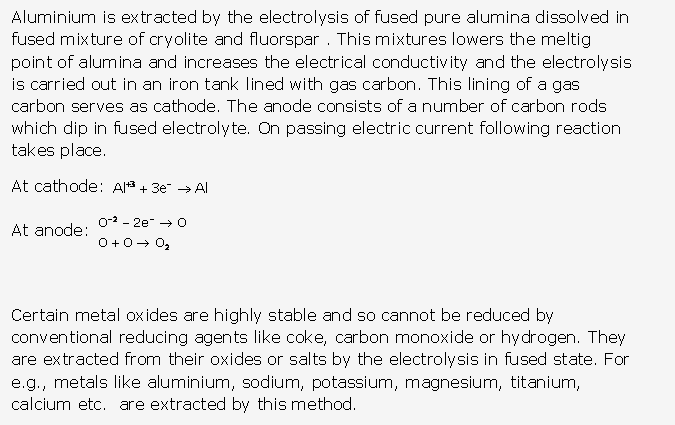
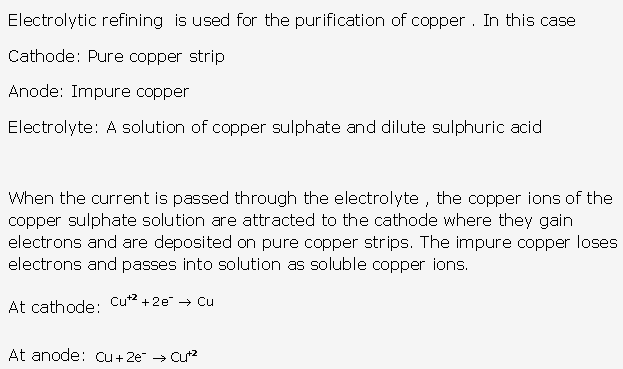
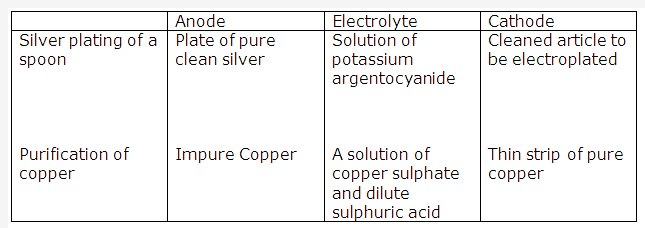
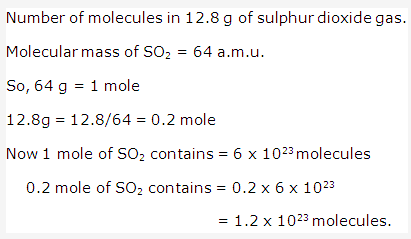
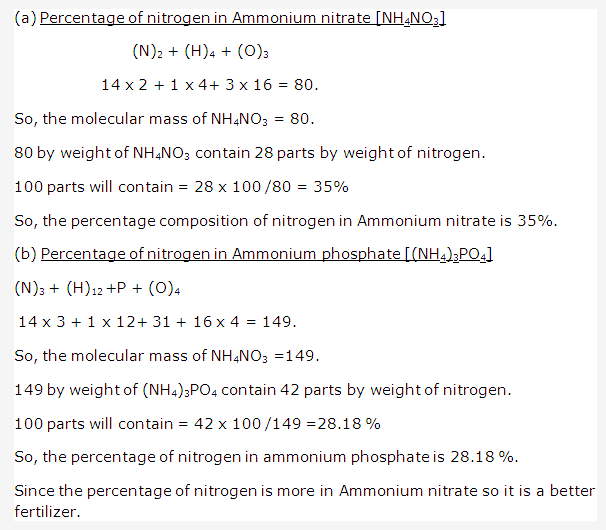
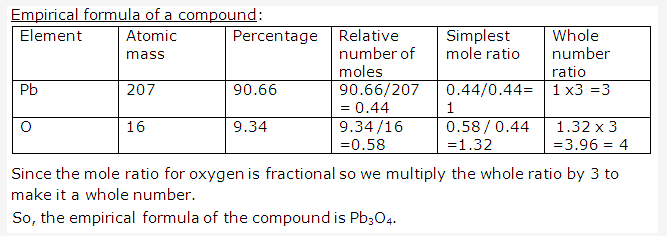
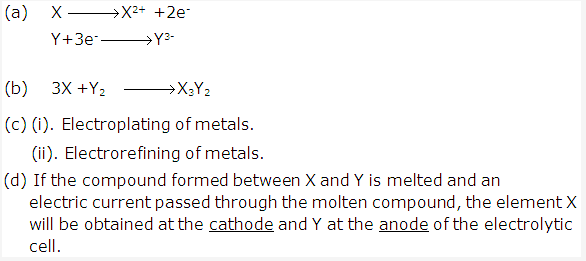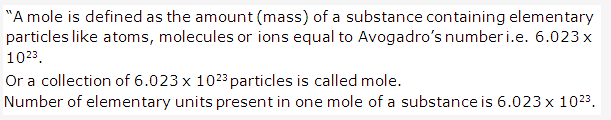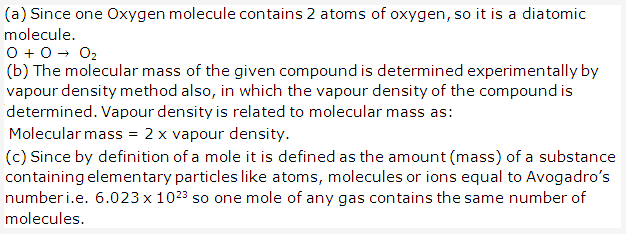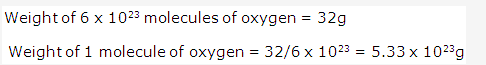Frank ICSE Solutions for Class 9 Chemistry – The Periodic Table
PAGE NO :132
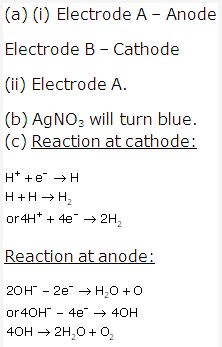
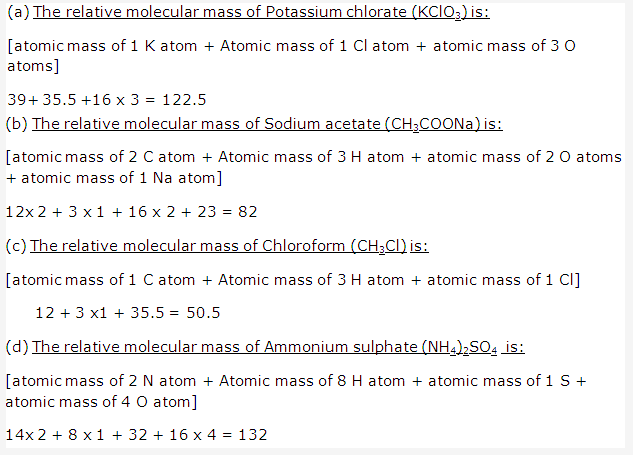
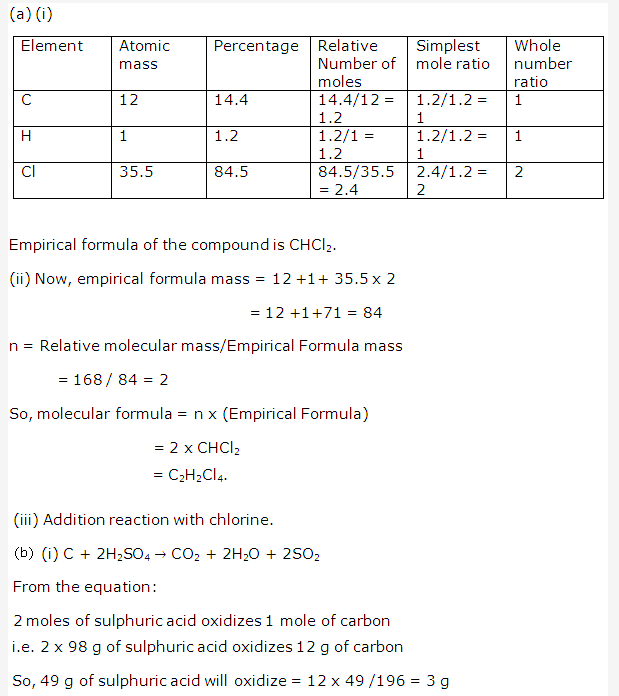
Solution 1:
The present form of periodic table has eighteen vertical columns called ‘groups’. These groups are numbered from 1 to 18.
Solution 2:
Seven horizontal rows in periodic table are called periods.These are numbered as 1, 2, 3, 4, 5, 6 and 7 from top to bottom.
Solution 3:
Certain chemically similar elements are arranged into set of three elements are called triads. When these elements are arranged in order of their atomic mass, the atomic mass of the middle element is approximately average of the atomic masses of the other two.
For Example – Lithium, Sodium, Potassium
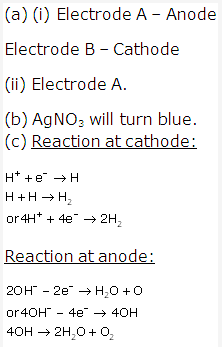
Solution 4:

Solution 5:
- (a) When elements are arranged in increasing order of their atomic masses, the eighth element resembles the first in physical and chemical properties just like the eighth note on a musical scale resembles the first note.
- (b) It is based on the notes of the musical scale.
- (c) No, the law of octaves was not valid beyond calcium i.e. not valid for heavier elements having atomic no. greater than ’40’.
- (d) There are 6 elements in between A and B.
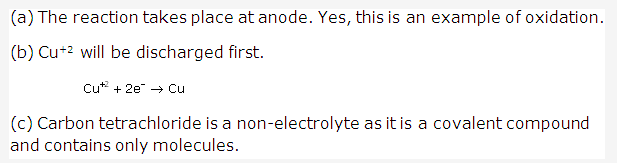
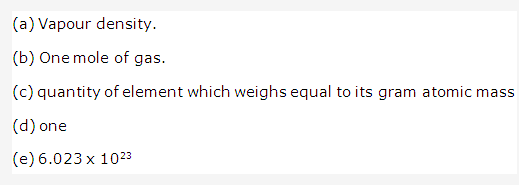
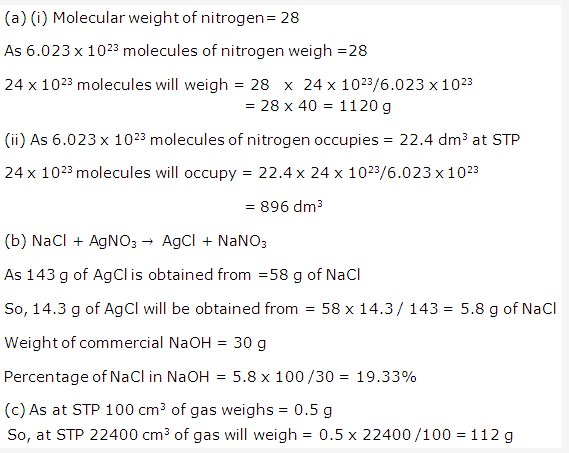
Solution 6:
Important conclusions of Newland’s law of octaves are-
- It was the first logical attempt to classify elements on the basis of atomic masses.
- Periodicity of elements was recognized for the first time.
Solution 7:
The physical and chemical properties of elements are the periodic functions of their atomic masses.
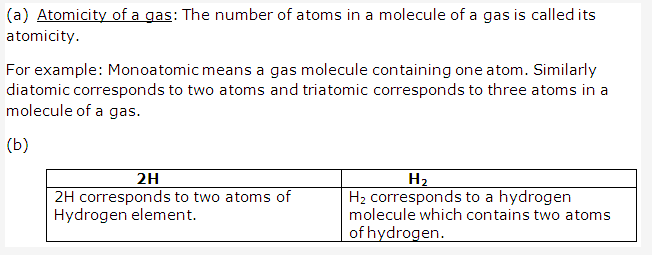
Solution 8:
- It helps to understand that the properties of elements are related with some fundamental unit of all elements.
- It simplifies and systemizes the study of the properties of various elements and their compounds.
- It helps to understand the relationship between the different types of elements.
- It helps to understand the cause of gradual change in properties from one element to another.
Solution 9:
The number of elements in various periods are-
Period 1 – 2
Period 2 – 8
Period 3 – 8
Solution 10:
Hydrogen and Helium are the elements of period 1.
Solution 11:
At the end of the period 2 and period 3 the outer shell of the elements are fully filled i.e. they have complete octet.
Solution 12:
The element of group II (2) is likely to be metallic in character.
Solution 13:
Metallic character increases on moving down the group. Therefore, the element that is placed at the end of the group will have the greatest metallic character.
Solution 14:
- (a) Two alkali metals and their groups and period are-
Lithium – First group and second period.
Sodium – First group and third period. - (b) Two alkaline earth metals with their groups and periods are-
Beryllium – Second group and Second period
Magnesium – Second group and Third period - (c) Halogens belong to Group 17.
- (d) Elements of groups from 3 to 12 are called as transition elements.
- (e) Helium gas has duplet arrangement of electrons.
- (f) Germanium is a metalloid of group 14.
- (g) The bond formed between the atoms of an element of group 2 and group 16 is called as electrovalent or ionic bond.
- (h) The bond formed between the atoms an element of group 14 and group 17 is called as covalent bond.
- (i) Lithium and Magnesium are the elements which are showing a diagonal relationship.
- (j) In Group 18 all elements are gases.
PAGE NO :133
Solution 15:
- (a) Newland, Newland law of Octaves
- (b) atomic masses
- (c) 18, 7
- (d) unstable
- (e) Atomic number, Moseley
- (f) similar outer electronic configuration at certain regular intervals
- (g) decreases
- (h) 2 electrons
- (i) different
Solution 16:
- (a) False
- (b) True
- (c) True
- (d) False
- (e) False
- (f) False
- (g) False
Solution 17:
- (a) A – 2, 1
B – 2, 3
C – 2, 8, 2
D – 2, 8, 8
E – 2, 8, 8, 2 - (b) B is placed right to A.
- (c) C and E belongs to same group.
- (d) The formula for sulphate of C is CSO4.
- (e) C has a smaller size atom.
- (f) Sodium is placed below A.
- (g) Helium is the first element of the group to which D belongs.
- (h) The formula for chloride of B is BCl3.
- (i) A2O is the formula of oxide of A.
- (j) Fluorine is the non-metallic element which belongs to the same period as A.
Solution 18:
- (a) Properties of Germanium i.e. also called as eka-silicon were predicted on the basis of its position in Mendeleev’s periodic table.
- (b) Beryllium and Gold are the two elements whose atomic weights were corrected on the basis of their positions in Mendeleev’s periodic table.
- (c) There were 63 elements known at the time of Mendeleev’s classification of elements.
PAGE NO :134
Solution 19:
Merits of Mendeleev’s classification of elements are:-
- Systematic study of the element: Elements could now be studied as groups or families rather than individuals. The Mendeleev’s periodic table simplified and systemized the study of the elements and their compounds.
- Prediction of new elements: While arranging the elements, in increasing order of atomic mass, Mendeleev left some blanks for the elements that were not discovered at that time. Though unknown, he was able to predict their properties more or less accurately.He named 3 of such elements as eka-boron, eka-aluminium, eka-silicon. He named them, as they were below boron,aluminium and silicon in the respective sub-groups.Eka-boron was later named as scandium, eka-aluminium as gallium and eka-silicon as germanium.
- Correction of atomic masses: The Mendeleev’s periodic table helped in correcting the atomic masses of elements based on their position in periodic table. Example-Atomic masses of Be was corrected from 13.5 to 9.
Solution 20:
Mendeleev left some gaps in his periodic table of elements for the elements that were not discovered at that time. Though they were unknown, he was able to predict their properties more or less accurately at that time.
Example – He named eka-boron, eka- aluminium and eka-silicon to some elements as they were just below boron, aluminium and silicon in the respective sub-groups. Eka-boron was later named as scandium, eka-aluminium as gallium and eka-silicon as germanium.
Solution 21:
The main characteristic of the last elements in the periodic table are-
- Their octet is attained.
- They are inert in nature.
The general name of such elements is noble gas or inert elements.
Solution 22:
- (a) The physical and chemical properties of elements are the periodic functions of their atomic numbers.
- (b) There are 7 periods and 18 groups in the long form of the periodic table.
- (c) The number of elements in each period is-
1 period-2
2 period-8
3 period-8
4 period-18
5 period-18
6 period-32
7 period-Incomplete
Solution 23:
- (a) X belongs to 17th group.
- (b) The period number of X is 3.
- (c) In atom of X there are 7 valence electrons.
- (d) Valency of X is 1.
- (e) It is non-metal.
- (f) The element right to it is Argon and the element left to it is Sulphur.
- (g) The atomic number of the elements above to it is 9 and below to it is 35 in the periodic table.
Solution 24:
- (a) The nature of bond will be ionic in the compound XY.
- (b)
- Y and Z will form covalent bond.
- X and Z will form ionic compound.
Solution 25:
- (a) There would be 2 electrons in the outermost shell of the element X.
- (b) Element X belong to second group of the periodic table.
- (c) When X reacts with chlorine, the compound formed of the formula XCl2.