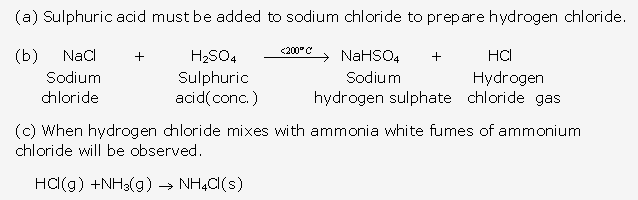
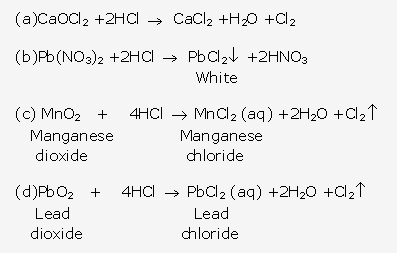
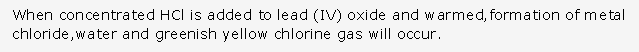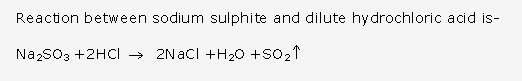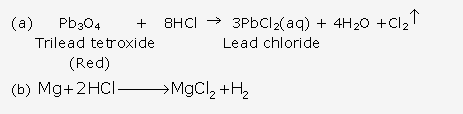Frank ICSE Solutions for Class 10 Chemistry – Metallurgy
PAGE NO : 152
Solution 9:


Solution 10:
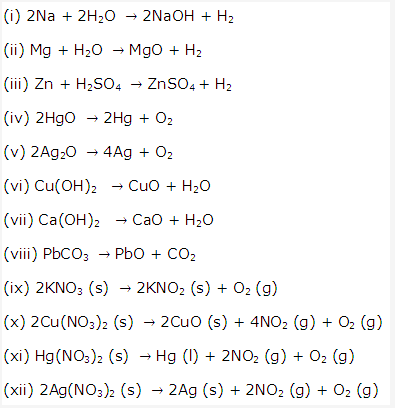
Solution 11:

Solution 12:
- Aqueous solution of sodium chloride is not used for electrolytic reduction of sodium metal because sodium metal formed at cathode after discharge of sodium ions( at cathode) will react with water to form alkali NaOH.
- For the reduction of a metal oxide a reducing agent other than carbon is carbon monoxide (CO).
Solution 13:
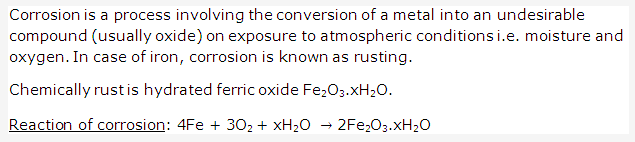
PAGE NO : 169
Solution 1:
- Gold and Platinum.
- Charge.
- Gangue.
- Flux.
- Calcination.
- Roasting.
- Iron pyrites.
- Bauxite.
- Cryolite, aluminium fluoride, Calcium fluoride.
- Cathode: inner lining of gas-carbon of the electrolytic cell.
Anode: Thick carbon rods dipping into the fused electrolytes. - Thermite welding.
- Copper and silver.
- Aluminium, Iron.
- platinum and gold
- sodium and potassium
Solution 2:
- Zinc is used in galvanization and dry cells because zinc coating protects the iron from corrosion as it is more electropositive than iron hence it would be attacked first.
- Nitric acid can be stored in aluminium containers because it do not attack aluminium. It renders aluminium passive due to the formation of an oxide film on surface of aluminum.
- Aluminium oxide cannot be reduced by carbon because it is comparatively high in electrochemical series hence more reactive than carbon.
- A neutral gas other than oxygen is formed at the anode during electrolysis of fused alumina because the oxygen gas formed at the anode oxidizes the carbon of the anode to carbon dioxide.
- Extraction of aluminium was very difficult in the beginning because it was very expensive.
- Carbon anodes are used in the electrolytic extraction of aluminium because carbon in the form of graphite is a good conductor of electricity.
- Galvanized metal ions should not be used for storing food as food acids may react with the zinc coating and cause food poisoning.
Solution 3:
- Mineral: The naturally occurring compounds of metals which are generally mixed with earthy such as soil, sand, limestone and rocks are known as minerals.
- Ore: Those minerals from which a metal can be extracted profitably are called ores.
- Gangue: The rocky impurities like (SiO2) present in an ore are called gangue.
- Charge: The mixture of materials fed into a furnace to extract a metal is called charge.
- Flux: The substance added to get rid of gangue in the extraction of metal is called flux.
- Slag: The product obtained by the combination of gangue with flux is called slag.
PAGE NO : 170
Solution 4:
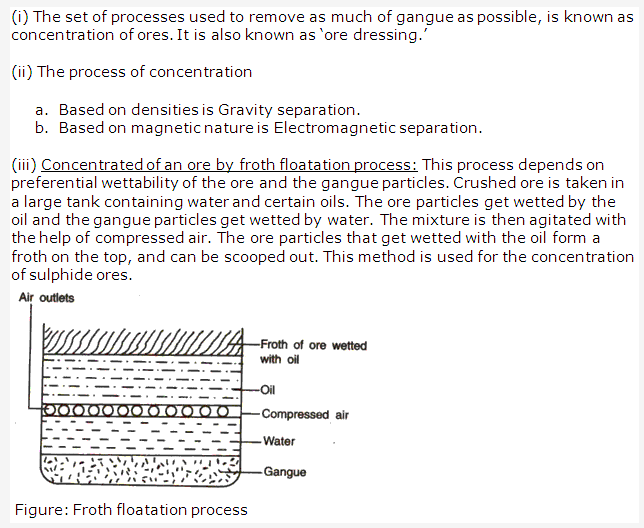
Solution 5:
- Mercury.
- Silver.
- Zinc.
- Aluminium.
Solution 6:
As we know that minerals are the naturally occurring compounds of metals which are generally mixed with earthy such as soil, sand, limestone and rocks while ores are those minerals from which a metal can be extracted profitably.
Hence “All ores are minerals but all minerals are not ores”.
Solution 7:
- Iron: Haematite(Fe2O3) and Magnetite (Fe3O4).
- Zinc: Zinc blende (ZnS) and Calamine (ZnCO3).
- Aluminium:Bauxite(Al2O3) and Cryolite (AlF3.3NaF).
Solution 8:
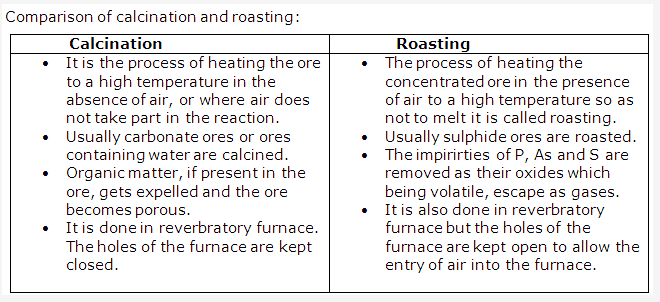
Solution 9:
Refining of metals: It is the further purification of metals obtained by reduction process to remove all the impurities.
Depending upon the nature of metal, nature of impurities and purpose for which metal is to be used. The three methods used for refining are:
- Liquation.
- Distillation.
- Electrolytic refining.
Solution 10:

Solution 11:

Solution 12:

Solution 13:
Cryolite acts as a solvent for the electrolytic mixture in the electrolytic reduction of alumina in the Hall’s process.
Solution 14:
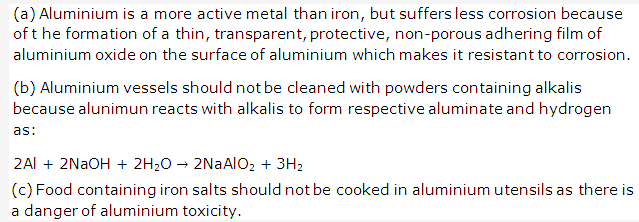
Solution 15:
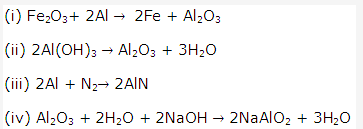
Solution 16:
An alloy is a homogeneous mixture of two or more metals fused together and then solidified.
Alloys are made because they have many salient features:
- Tensile strength.
- Strength.
- Electrical hardness.
Solution 17:
The properties of alloys which are different from constituent metals are:
- Alloys are stronger and harder than the metals of which they are made.
- Alloys are more resistant to corrosion.
Solution 18:
Amalgam: A mixture or an alloy of mercury with a number of metals or non-metals is known as amalgams. An amalgam may be liquid such as Na/Hg or a solid like Zn/Hg.
- Iron does not form amalgam.
- Dental amalgam which is a mixture of mercury with a silver tin alloy is used for dental fillings.
Solution 19:
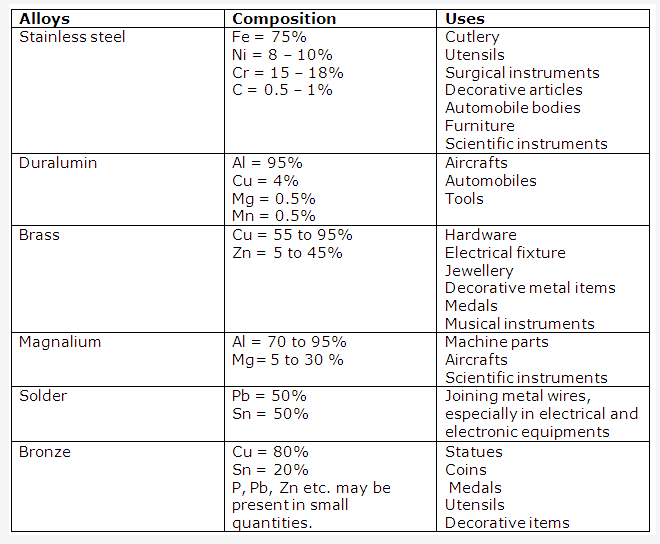
Solution 20:
- Galvanization protects iron from rusting because in galvanization coating of zinc is done over iron articles and zinc being more electropositive would be attacked preferably than iron.
- Stainless steel is more useful than steel as it is harder, has high tensile strength, more lustre, more resistance to corrosion and many chemicals.
- Aluminium is extensively used for making aircraft parts because of features like high tensile strength, corrosion resistance light but hard and tough.
- Cold water has no action on aluminium while burning aluminium decomposes steam.
Solution 21:
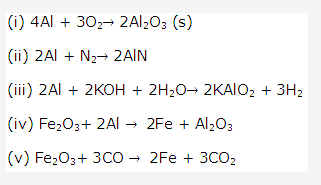
PAGE NO : 171
Solution 1991-1:
- Iron having a coating of zinc is called galvanized iron.
- iron which cannot be easily acted upon by acidsis called as passive iron. Galvanized iron is called passive iron since coating of zinc protects the iron from corrosion as zinc is more electropositive and so would be attacked first.
Solution 1991-2:
Zinc amalgam which is a mixture of zinc and mercury.
Solution 1992-1:
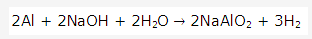
Solution 1992-3:
- Nitrogen.
- Iodine
- Bromine
- Carbon in the form of graphite
Solution 1992-:
- Cryolite is Na3AlF6 and its chemical name is Sodium aluminium fluoride.
- Cryolite is used in the electrolysis of alumina. The function of cryolite is to
- Reduce melting point of alumina
- Make molten alumina a good conductor of electricity
Solution 1993-1:
Gold.
Solution 1993-2:

Solution 1994-1:
Reactivity of metals with water Sodium, calcium, magnesium, iron
Solution 1994-2:

Solution 1995-1:
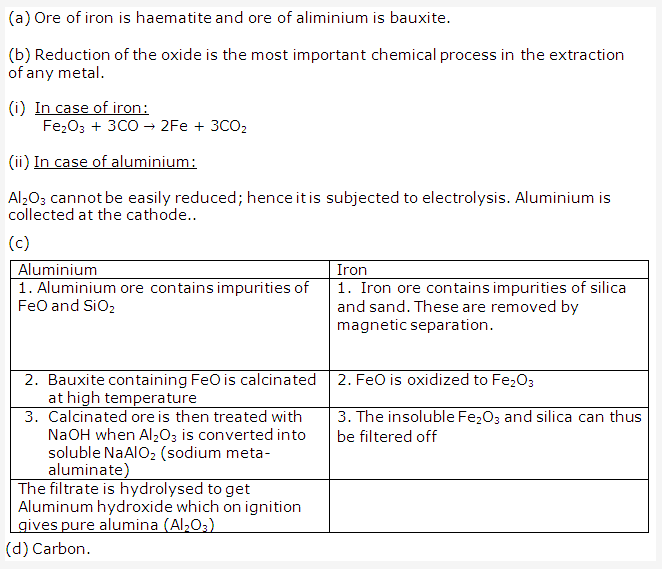
Solution 1995-2:
Zinc amalgam.
PAGE NO : 172
Solution 1996-1:

Solution 1996-2:

Solution 1996-3:
An alloy is a homogeneous mixture of two or more metals fused together and then solidified.
- The special property of duralumin is:
- Light but hard
- Resistant to corrosion
- Ductile
- Type metal = Hard
Solution 1997-1:
Chromium and nickel is added to steel to make it stainless steel.
Solution 1997-2:
Ore: Those minerals from which a metal can be extracted profitably are called ores. For example bauxite ore is used to extract aluminium metal, hematite ore is used to extract iron metal.
Solution 1998-1:
- good, poor.
- non-malleable.
- form negative ions.
- basic oxides.
Solution 1998-2:
- Mercury.
- Graphite.
Solution 1998-3:
Metals have 1, 2, 3 valence electrons while non-metals have 4, 5, 6 or 7 valence electrons.
Solution 1999-1:
Magnesium oxide, iron (II) oxide, lead (II) oxide and then copper (II) oxide.
Solution 1999-2:

Solution 1999-3:
PAGE NO : 173
Solution 2000-1:
- Blue
- Red
- Hydrogen
- acidic, acidic
- graphite.
Solution 2001-1:
- Copper
- Iron
- Zinc
- Magnesium
Solution 2001-2:
Sodium > magnesium > Zinc > Iron > Copper
Solution 2002-1:

Solution 2002-2:

Solution 2003-1:
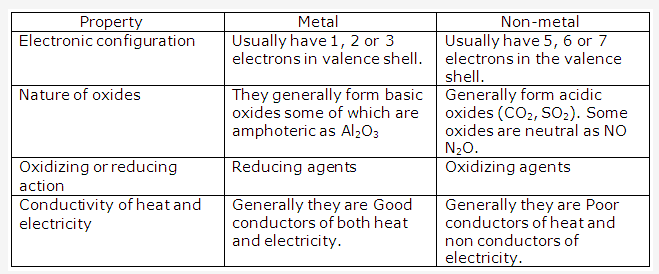
Solution 2004-1:
Iodine is a non- metal that has a metallic luster and sublimes on heating.
Solution 2004-2:
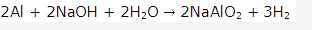
Solution 2004-3:
Zinc blende (ZnS)
Solution 2004-4:
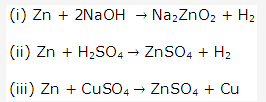
Solution 2004-5:
Galvanization.
Solution 2004-6:

Solution 2005-1:
- (i) B, D F
(ii) A, C E - (i) Sodium hydroxide solution
(ii) Cryolite - Na3AlF6
PAGE NO : 175
Solution 2005-2:
- For stainless steel: iron, chromium
- For brass: Copper and zinc.
Solution 2006-1:
- Mercury.
- Cryolite.
- Roasting.
- Calcium silicate.
- Zone of heat absorption.
Solution 2007-1:
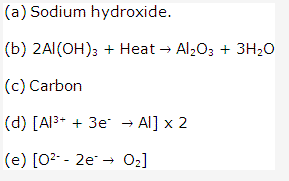
Solution 2008-1:
(b)
PAGE NO : 176
Solution 2009-1:
- Carbon as it forms very large number of compounds while the rest do not.
- Mercury as it is a liquid metal while the rest aresolid.
Solution 2009-2:
- Copper reacts with concentrated nitric acid to produce nitrogen dioxide.
- Bauxite is the chief ore of aluminium.
Solution 2009-3:
- A is cathode and B is anode.
- Molten fluorides of Al, Na and Ba.
- Graphite rods.
Solution 2009-4:
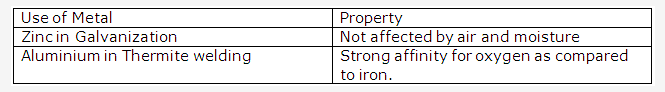
ChemistryBiologyPhysicsMaths











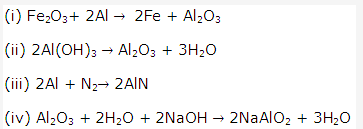

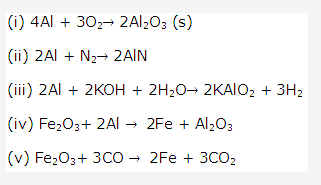









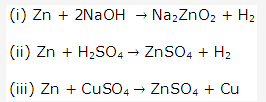

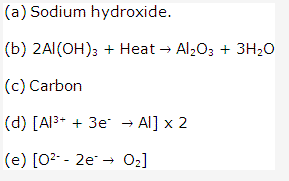

 f
f