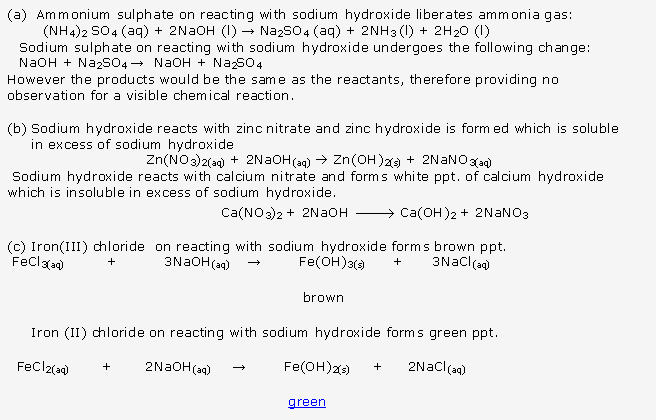
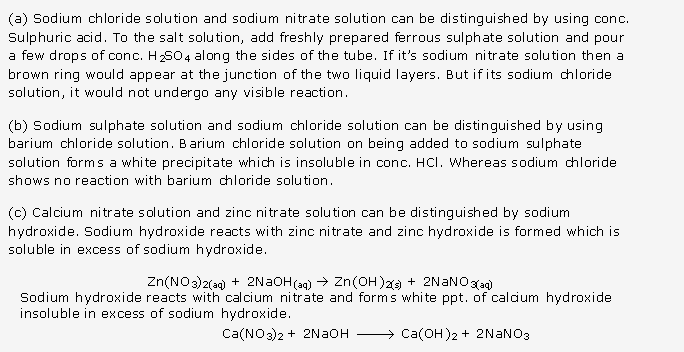
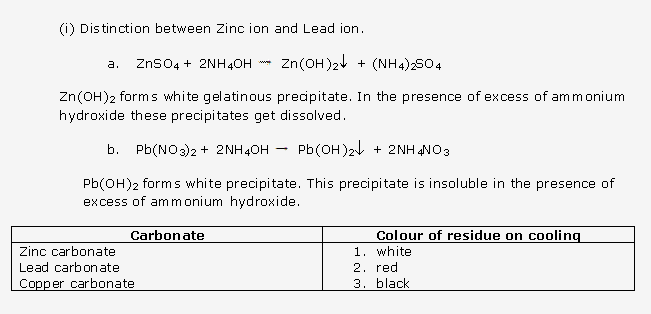
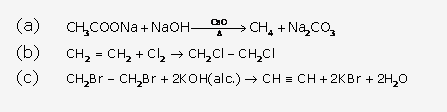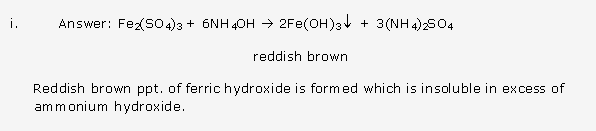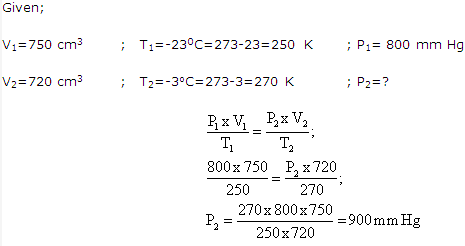Frank ICSE Solutions for Class 10 Chemistry – Periodic Properties and Variation of Properties
PAGE NO : 16
Solution 1:
Modern periodic law states that the physical and chemical properties of elements are a periodic function of their atomic numbers i.e., if the elements are arranged in the order of their atomic numbers, the elements with similar properties are repeated after definite regular intervals.
Concept Insight: The elements are characterized by their atomic number as well as atomic weight. Modern periodic law uses atomic number which is number of protons or number of electrons present in an atom of an element.
Solution 2:
Modern periodic table consists of eighteen groups and seven periods.
Concept Insight: Classification of elements on the basis of increasing atomic number is known as Modern Periodic Table. The vertical columns are called groups and the horizontal rows are called periods.
Solution 3:
The recurrence of similar properties of elements after certain regular intervals when they are arranged in the order of increasing atomic numbers is called periodicity.
Concept Insight: Periodicity in properties is due to the repetition of similar outer electronic configuration of elements at certain regular intervals.
Solution 4:
In general, the elements belonging to a group have the same number of valence electrons .For example, all the group 1 elements have valency one since they have only one electron in their outermost shell.
In general, the elements belonging to a period do not have same valency but their valence shell remains the same. For example, second period has 8 elements with atomic number 3 to 10 but in all of them the valence electrons are present in shell number two.
Concept Insight: For elements in a group the number of electrons present in the outermost shell is the same and therefore the elements have same valency and or elements in a period number of electrons present in the outermost shell of elements in a period increase from left to right but the shell does remains the same.
Solution 5:
Fluorine has lower electron affinity than chlorine because of the small size of fluorine which results in stronger repulsion between the electron and the electrons already present in the atom of fluorine. Hence the energy released in accepting an electron is lesser in fluorine than that of chlorine.
Concept Insight: For answering this question you should recall that electron affinity is the energy released when an electron is added to an isolated gaseous atom to form an anion. Due to small size of fluorine atom, the valence shell is already crowded, hence when an electron is added to a fluorine atom in gaseous state there occurs strong repulsion between the added electron and those already present in the atom hence less amount of energy is released.
Solution 6:
- The element with highest first ionization energy: Neon (Ne)
- The element with highest electro negativity: Fluorine (F)
- The element with largest atomic size: Lithium (Li)
- The most reactive non-metal: Fluorine
- The most reactive metal: Lithium
- Concept Insight:
- Neon has highest ionization energy since it is a noble gas and has its octet complete which makes it very stable.
- Fluorine has highest electronegativity as we know that electro negativity increases along a period due to decreasing atomic size and increasing nuclear charge.
- Lithium has the largest atomic size since it is an alkali metal i.e., belongs to group 1 and we know that as we move from left to right in a period atomic size decreases. So lithium has largest size while fluorine has smallest size in second period.
- Fluorine is most reactive non metal as it requires only one electron to complete its octet and become stable.
- Lithium is the most reactive metal as it can complete its octet by losing its single electron present in its outermost shell.
PAGE NO : 17
Solution 7:
- The most metallic element will be found at C.
- The most non-metallic element will be found at D.
Concept Insight: For answering this question you should recall metallic character increases down the group and also increases with the increasing size of the atom. Since elements of group 1 has largest atomic sizes among all the elements of periodic table so the most metallic element belongs to group 1.
Similarly, non-metallic character decreases down the group and increases with the decreasing size of atom.
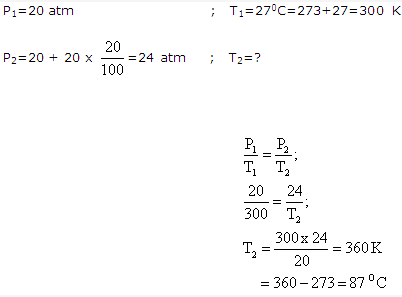
Solution 8:
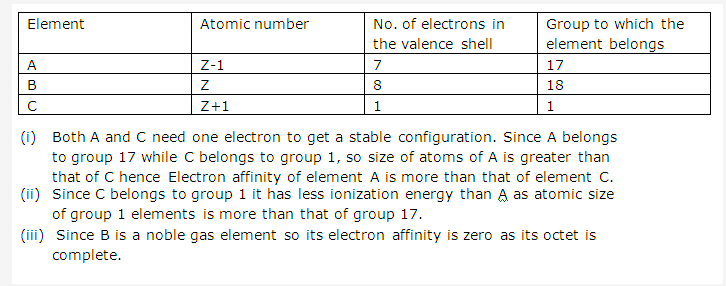
Solution 9:
- 18, 7.
- First.
- Seventeen.
- Electron affinity.
- Decrease, increase
- Fluorine
- Zero.
Solution 10:
- False.
- True.
- True.
- True.
- True.
- True.
Concept Insight: (i) There is inverse relation between atomic size and electron affinity. More is the size, less is the electron affinity and vice versa because more is the size of atom, more is the distance between the nucleus and last shell to which electron enters. This results in decrease in force of attraction between the nucleus and incoming electron and hence the electron affinity decreases. Fluorine has smaller size than chlorine so it must have less electron affinity than chlorine.
Solution 11:
- Li < Be < B
- I < Br < F < Cl
- SiO2 < P2O5 < SO3 < Cl2O7
- I+ < I < I–
Concept Insight:
- Li, Be, B belongs to second period and ionization energy increase as we move left to right in a period due to increased nuclear charge and decrease of atomic size.
- Electron affinity decreases down the group due to increase in atomic size as it results in more distance between nucleus and last shell to which incoming electron enters. Hence, incoming electron feels less attraction from the nucleus.
- In a period, acidic nature of oxide increases.
- Size of a cation is always smaller than the corresponding atom due to decrease in number of electrons and increase in effective nuclear charge i.e., greater force of attraction by the nucleus on the electrons.
Size of an anion is always more than the corresponding atom due to decrease in effective nuclear charge i.e., lesser force of attraction by the nucleus o the electrons.
Solution 12:
The statement that in each period, the atomic size gradually decreases with increase in atomic number means that as move from left to right in a period, nuclear charge increases by one unit in each succeeding element while the number of shells remains the same. Due to this increased nuclear charge, the electrons of all the shells are pulled closer to the nucleus thereby bringing the outer most shell closer to the nucleus. With the result, the atomic size decreases across a period.
For example, in the second period from lithium to fluorine, lithium has the largest size while fluorine has the smallest size.
Solution 13:
In the case of noble gases or inert gases there are exceptions and the atomic radius or size of the elements are greater than the other elements of the period to which these elements belong.
PAGE NO : 18
Solution 14:
As we move down a group, the atomic radii increase because a new shell is added at each succeeding element though the number of electrons in the outer most shell remains the same. Thus, the atomic size of elements increases in size downward.
Although nuclear charge also increases in going down the group but the effect of nuclear charge on atomic size is much less than the increase due to addition of a new shell.
In group 17, the atomic size follows the trend:
F < Cl < Br < I
Solution 15:
The elements of third period are:
Na, Mg, Al, Si, P, S, Cl, Ar
The most metallic element is sodium i.e., Na and the most non-metallic element is chlorine i.e., Cl.
Concept Insight:
In a period, metallic character decreases on moving from left to right because of decrease in size of atom due to which elements cannot lose electron easily.
Solution 16:

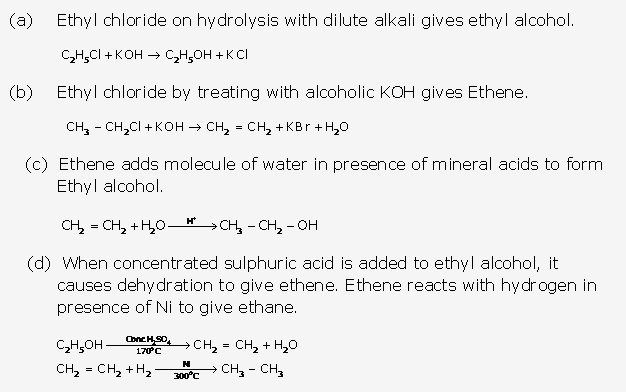
Solution 17:
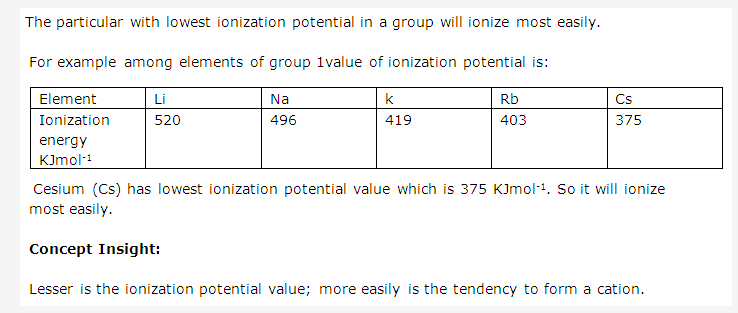
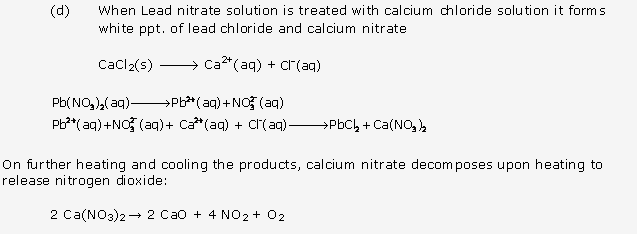
Solution 18:
Electron affinity is the energy released when an electron is added to an isolated gaseous atom to form the negative ion (anion).
Unit: Its units are electron volt (eV).
Its SI units are Kilojoules per mole(KJmol)-1
Solution 19:
Out of A and B, A will ionize more easily to form a negative anion because of the high value of electron affinity, energy released during addition of electron will be high hence the resulting anion formed will be more stable than the corresponding atom.
Solution 20:
- Larger the atomic size, farther is the valence electron from the nucleus and lesser is the pull exerted on it. As a result, electron can be easily removed from the valence shell and hence more metallic is the element.
- Halogens need only one electron to complete their octet and become stable their atomic size is very less hence the distance between their last shell and nucleus is very less, as a result the force of attraction between the nucleus and the incoming electron is less and hence the electron affinity is high for halogens.
- When an atom loses or gain electron to form ion, the number of electrons present in the outermost shell also changes. Corresponding to that effective nuclear charge on the changed number of electrons also change which further changes the size of an atom as there is inverse relation between effective nuclear charge and size of atom.
- K and Li belongs to group 1 i.e., metals and we know that for metals chemical reactivity of elements increases down the group because chemical reactivity increases as electropositive or metallic character increases.
- The electronegativity of chlorine is higher than sulphur because both of them belong to third group and chlorine follows sulphur. We know that, within a period electronegativity increases as we move from left to right because of decrease in atomic size and increase in nuclear charge.
- Group 17 elements are non metals because they have 7 electrons in their valence shell and ionize by accepting 1 electron to form an anion.
For example group 17 elements F, Cl, Br and I all have 7 electrons in their valence shell and ionize by accepting 1 electron to form F–, Cl–, Br– and I–.
Group 1 elements are metals because they have tendency to lose the one electron present in their valence shell and form positive ion.
For example, group 1 elements Li, Na, K, Rb, Cs have tendency to lose the one electron present in their valence shell and form positive ions Li+., Na+., K+., Rb+.and Cs+.
Solution 21:
- (C) i.e. 2, 8, 2 because it has only 2 electrons in its valence shell which can be lost to form a di positive cation.
- (C) i.e. 0.72, 0.72
- (d) i.e. element forms basic oxide because the element is a metal as it has valency 1.
- (a) i.e. F because it belongs to group 17 whose elements have valency 7 and thus requires only 1 electron to complete their octet.
Solution 2000-1:
- Number of elements in period 1 = 2
In period 2 = 8
In period 3 = 8. - Elements in period 1 are Hydrogen (H) and Helium (He).
- Atomic size of elements decreases on moving from left to right in a period.
Solution 2000-2:
- The elements at the end of period 2 and period 3 both have their outermost shell complete and belong to noble gases.
- An element in group 7 is likely to be non metallic in character since group 7 element will have 7 electrons in its valence shell.
- Metallic.
PAGE NO : 19
Solution 2001-1:
- Atomic number.
- Period, non-metallic.
- More.
- Number of outer electrons.
Solution 2002-1:
A group is a vertical column of elements having the same number of valence electrons and same valency in the periodic table. There are 18 groups in the periodic table.
Solution 2002-2:
Within a group the element with the greatest metallic character and largest size is expected to be present at the bottom of the group.
Solution 2002-3:
Ionization potential decreases down the group because atomic size increases down the group which decreases the effective nuclear charge over the valence electron which further can now be removed easily.
Solution 2002-4:
There are 8 elements in period 2.
Solution 2003-1:
- Al2(SO4)3
- Covalent.
- The elements of group VIIA all have same number of electrons in their valence shell and same valency.
- Neon
- 8 electrons are present in the valence shell of the element with atomic number 18.
- Electron affinity.
- Electronic configuration of element in the third period which gains one electron to become an anion is 2, 8, 7.
- Decreases, number of valence shell electrons/outermost shell electrons, valence shell/ outermost shell.
PAGE NO : 20
Solution 2004-1:
- Na Mg Al Si P S Cl.
- (a) lower, higher.
- remains the same.
Solution 2005-1:
- b
- d
- c
- a
- c
Solution 2006-1:
- Second period.
- Nitrogen. It should be placed between oxygen andcarbon.
- Beryllium < nitrogen < fluorine
- Fluorine (F)
Solution 2007-1:
- Thallium
- Boron
- 3
- BCl3
- The elements in the group to the right of this boron group will be less metallic in character because on moving to the right of the periodic table metallic character decreases as ionization energy deceases and tendency to lose electron also decreases.
Solution 2008-1:
- False
- True
- False
- True
Solution 2008-2:
- (i) First element in period 2 is Lithium and last element is Neon.
(ii)Atomic size increases on moving from top to bottom of a group.
(iii)Chlorine among halogens has the greatest electron affinity.
(iv) All elements in group 7 have same number of valence shell electrons. - (i) metallic
(ii) smallest - (i) Ba i.e. Barium will form ion most readily since it is at the bottom of a group its ionization energy is low because its atomic size is more. Due to this effective atomic charge of nucleus over the valence shell electron is least and it can be removed easily.
(ii) Electro negativity of an element measures the capacity of an element to attract the shared pair of electrons in a bond towards itself.
Solution 2009-1:
(d) Fluorine
PAGE N0 : 22
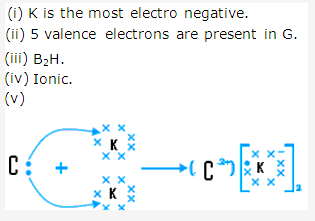
Solution 2009-2:
ChemistryBiologyPhysicsMaths



![]()
![]()
![]()







![]()








![]()