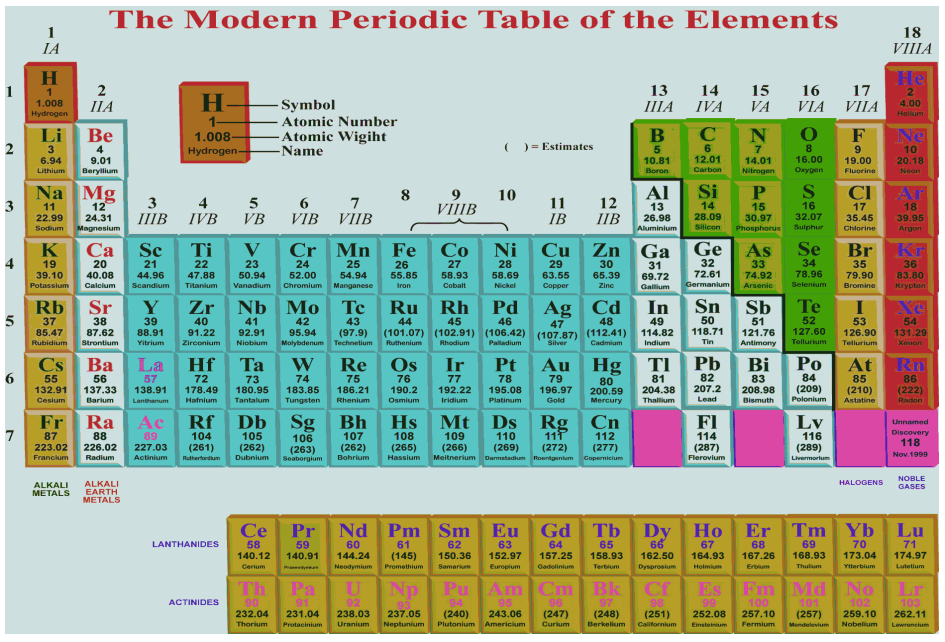
Modern Periodic Table and Its Significance
Modern Periodic Table :
Henry Moseley, an English physicist found that the atomic number (Z) was the fundamental property of an elements and not the atomic mass for classification of elements.
Modern Periodic Law :
‘‘Properties of elements are periodic functions of their atomic numbers, i.e., the number of protons or electrons present in the neutral atom of an element.’’
Long form of Periodic Table :
Arranged in increasing order of their atomic numbers.
The prediction of properties elements and their compounds can be made with precision. All drawbacks of Mendeleev’s Periodic Table vanish when the elements are arranged on the basis of increasing atomic numbers.
Elements in a Group :
(1) They show similar chemical properties due to similar outer electronic configuration, i.e., same number of valence electrons.
(2) They have gradation in properties due to gradually varying attraction of the nucleus and the outer valence electrons as we go down the group.
Main Features of the Long Form of the Periodic Table :
(1) It shows arrangement of elements based on modern periodic law.
(2) There are 18 vertical columns known as groups.
(3) There are 7 horizontal rows known as periods.
(4) Elements having similar outer electronic configurations, i.e., having same valence electrons have been placed in same groups, e.g.,
| Group-1 | ||||||||
| K | L | M | N | O | P | Q | ||
| H | (1) | 1 | ||||||
| Li | (3) | 2, | 1 | |||||
| Na | (11) | 2, | 8, | 1 | ||||
| K | (19) | 2, | 8, | 8, | 1 | |||
| Rb | (37) | 2, | 8, | 18, | 8, | 1 | ||
| Cs | (55) | 2, | 8, | 18, | 18, | 8, | 1 | |
| Fr | (87) | 2, | 8, | 18, | 32, | 18, | 8, | 1 |
| Group-2 | ||||||||
| K | L | M | N | O | P | Q | ||
| Be | (4) | 2 | 2 | |||||
| Mg | (12) | 2, | 8, | 2 | ||||
| Ca | (20) | 2, | 8, | 8, | 2 | |||
| Sr | (38) | 2, | 8, | 18, | 8, | 2 | ||
| Ba | (56) | 2, | 8, | 18, | 18, | 8, | 2 | |
| Ra | (88) | 2, | 8, | 18, | 32, | 18, | 8, | 2 |
| Group-13 | |||||||
| K | L | M | N | O | P | ||
| B | (5) | 2 | 3 | ||||
| Al | (13) | 2, | 8, | 3 | |||
| Ga | (31) | 2, | 8, | 18, | 3 | ||
| In | (49) | 2, | 8, | 18, | 18, | 3 | |
| Tl | (81) | 2, | 8, | 18, | 32, | 18, | 3 |
| Group-14 | |||||||
| K | L | M | N | O | P | ||
| C | (6) | 2 | 4 | ||||
| Si | (14) | 2, | 8, | 4 | |||
| Ge | (32) | 2, | 8, | 18, | 4 | ||
| Sn | (50) | 2, | 8, | 18, | 18, | 4 | |
| Pb | (82) | 2, | 8, | 18, | 32, | 18, | 4 |
| Group-15 | |||||||
| K | L | M | N | O | P | ||
| N | (7) | 2 | 5 | ||||
| P | (15) | 2, | 8, | 5 | |||
| As | (33) | 2, | 8, | 18, | 5 | ||
| Sb | (51) | 2, | 8, | 18, | 18, | 5 | |
| Bi | (83) | 2, | 8, | 18, | 32, | 18, | 5 |
| Group-16 | |||||||
| K | L | M | N | O | P | ||
| O | (8) | 2 | 6 | ||||
| S | (16) | 2, | 8, | 6 | |||
| Se | (34) | 2, | 8, | 18, | 6 | ||
| Te | (52) | 2, | 8, | 18, | 18, | 6 | |
| Po | (84) | 2, | 8, | 18, | 32, | 18, | 6 |
| Group-17 | |||||||
| K | L | M | N | O | P | ||
| F | (9) | 2 | 7 | ||||
| Cl | (17) | 2, | 8, | 7 | |||
| Br | (35) | 2, | 8, | 18, | 7 | ||
| I | (53) | 2, | 8, | 18, | 18, | 7 | |
| At | (85) | 2, | 8, | 18, | 32, | 18, | 7 |
| Group-18 | |||||||
| K | L | M | N | O | P | ||
| He | (2) | 2 | |||||
| Ne | (10) | 2, | 8 | ||||
| Ar | (18) | 2, | 8, | 8 | |||
| Kr | (36) | 2, | 8, | 18, | 8 | ||
| Xe | (54) | 2, | 8, | 18, | 18, | 8 | |
| Rn | (86) | 2, | 8, | 18, | 32, | 18, | 8 |
People also ask
- What is the periodic table of the elements?
- Physical and Chemical Properties of Group 1 Elements
- Physical and Chemical Properties of Group 18 Elements
- Physical and Chemical Properties of Group 17 Elements
- What do you mean by transition metals?
- What is the Need for Classification of Elements?
- How did Mendeleev Arrange the Periodic Table?
- Periodic Trends in Properties of Elements
- What is Valency and Atomic Radius?
- What are Metallic and Nonmetallic Properties?
(5) In periods, elements in which the number of electrons in the outermost shell increases gradually in step one are placed, e.g.,
Period 1 (K-shell) | H 1 | He 2 | ||||||
Second Period (K, L, shells) | Li(3) 2, 1 | Be(4) 2, 2 | B(5) 2, 3 | C(6) 2, 4 | N(7) 2, 5 | O(8) 2, 6 | F(9) 2, 7 | Ne(10) 2, 8 |
(6) Each group in the table signifies identical outer shell electronic configuration i.e., same valence electrons, e.g., group 1 has 1 valence electron, group 2 has 2 valence electrons, group 13 has 3, group 14 has 4 valence electrons.
(7) Each period starts with filling of new shell, e.g.,
1st Period – K shell (1st shell) starts filling with Hydrogen and ends at Helium.
2nd Period – L shell (2nd shell) starts filling from Li (3) upto Ne (10)
3rd Period – M shell (3rd shell) start filling from Na (11) upto Ar (18)
4th Period – N shell (4th shell) starts filling from K (19) upto Kr (36) and so on.
(8) The periodic table is divided in four blocks :
(a) s-block elements : Group 1 and 2 elements are called s-block elements.
(b) p-block elements : Group 13 to 18 elements are called p-block elements
(c) d-block elements : Group 3 to group 12 are called d-block elements or transition elements (in between s- block and p-block elements)
(d) f-block elements : The elements placed at the bottom of the periodic table are known as f-block elements. The fourteen elements after La(57) (Lanthanum) are called Lanthanoides and 14 elements after Actinium Ac (89) are called Actinoides.