Rusting (Corrosion) as a Redox Reaction
What is corrosion of a metal?
Corrosion of metal:
- When metals are exposed to their environment, they undergo corrosion. For example, after some time, a shiny aluminium pot will lose its shine, silverware will tarnish and an iron structure will rust.
- Corrosion of metal is a redox reaction in which a metal is oxidised naturally to its ions, resulting in partial or complete destruction of the metal.
- During corrosion, the metal atoms lose electrons to form positive ions.
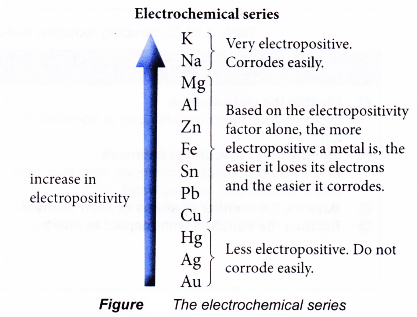
M → Mn+ + ne– - Some metals corrode more easily than others. How easily a metal corrodes depends on two factors:
(a) Electropositivity of metals
(b) Nature of the product of corrosion - Electropositivity of metals
- Nature of the product of corrosion
When a metal corrodes, it usually forms an oxide coating.
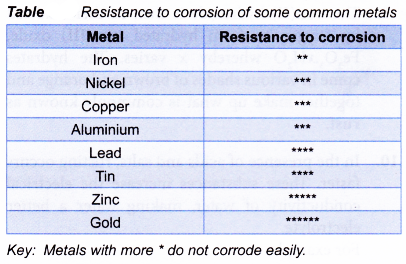
(a) The oxide coating of aluminium, for example, is tightly packed, non-porous and is firmly attached to the metal. It does not let water and air penetrate through it, protecting the aluminium underneath from further corrosion. This explains why aluminium is quite resistant to corrosion even though it is very electropositive. Other metals with similar protective oxide coating include nickel, chromium, tin, lead and zinc.
(b) The oxide coating of iron on the other hand, is not tightly packed, porous, weak and easily peels off. Thus, water and air can penetrate through the coating to further corrode the iron metal underneath it. - Table compares the resistance to corrosion of some common metals.
People also ask
- What is a redox reaction?
- Changing of iron(II) ions to iron(III) ions and vice versa
- Redox reaction in the displacement of metals from its salt solution
- Displacement of Halogen From Halide Solution
- Redox Reactions by Transfer of Electrons at a Distance
- The Reactivity Series of Metals Towards Oxygen
- Application of the reactivity series of metals in the extraction of metals
- Electrolytic and Chemical Cells
- Oxidation and Reduction in Electrolytic Cells
- Oxidation and Reduction in Chemical Cells
- How does a voltaic cell work?
Rusting as a redox reaction
- Rusting is the corrosion of iron. It is the most common corrosion of metal around.
- For iron to rust, oxygen and water must be present.
- Rusting is a redox reaction whereby oxygen acts as the oxidising agent and iron acts as the reducing agent.
- Figure shows the half-reactions of rusting.
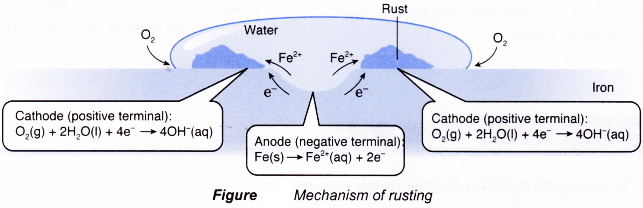
- The surface of iron at the middle of the water droplet serves as the anode, the electrode at which oxidation occurs. The iron atoms here lose electrons to form iron(II) ions.
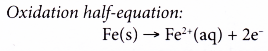
- The electrons flow to the edge of the water droplet, where there is plenty of dissolved oxygen. The iron surface there serves as the cathode, the electrode at which reduction occurs. Oxygen gains the electrons and is reduced to hydroxide ions.
- The iron(II) ions produced combine with the hydroxide ions to form iron(II) hydroxide.
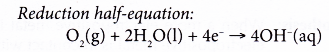
- Thus, the overall redox reaction is as follows:
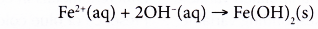
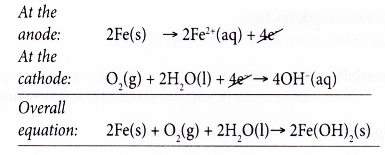
- The iron(II) hydroxide is then further oxidised by oxygen to form hydrated iron(III) oxide, Fe2O3.xH2O) whereby x varies. The hydrates come in various shades of brown and orange and together make up what is commonly known as rust.
- In the presence of acids and salts, rusting occurs faster. These substances increase the electrical conductivity of water, making water a better electrolyte.
For example:
(a) Iron structures such as bridges, fences and cars at coastal areas rust faster due to the presence of salts in the coastal breeze.
(b) Iron structures in industrial areas rust quickly as these areas have air polluted with acidic gases such as sulphur dioxide and nitrogen oxides.
Other metals and rusting of iron
- When two metals are in contact with each other, the more electropositive metal will corrode first. This is simply because the more electropositive metal can lose its electrons more readily than the less electropositive metal.
- So, when iron is in contact with a more electropositive metal, rusting of iron is prevented or inhibited.
- For example, magnesium is more electropositive than iron. So, when iron is in contact with magnesium, magnesium corrodes or is oxidised instead of iron.
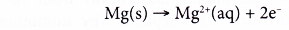
- On the other hand, when iron is in contact with a less electropositive metal, rusting of iron is speeded up.
- For example, copper is less electropositive than iron. Therefore, when iron is in contact with copper, iron rusts faster.
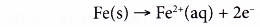
- The further apart the metals are in the electrochemical series, the faster the more electropositive metal corrodes. For example, iron rusts faster when in contact with copper than when it is in contact with tin.

What are some ways of preventing corrosion of metals?
Controlling rusting:
Generally, there are three main ways to control rusting.
1. Using protective coating
The protective coating prevents water and air from reaching the surface of iron. Various materials can be used as the protective coating, depending on the costing and usage of the iron items.
- Oil and grease are used for moving parts of engine.
- Paints are used for items that are not easily scratched such as cars, ships, bridges, railings and gates. For example, most modern cars have a few layers of anti-rust coating and paints on them. Some pots and plates have enamel-paint coated on them.
- Plastics are used for light items such as clothes hanger and wire fences.
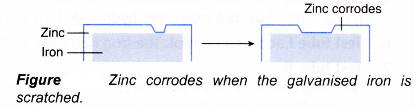
- Galvanising (zinc plating) involves coating an iron or steel sheet with a thin layer of zinc. This is done by dipping the iron into molten zinc.
- Galvanising is used on objects that are exposed to the atmosphere, such as iron roofing, water tanks and iron rubbish bins.
- Galvanised iron is prevented from rusting in two ways. Firstly, the zinc layer provides a protective oxide coating.
- Secondly, when the galvanised iron is scratched, zinc corrodes first instead of iron because zinc is more electropositive than iron.
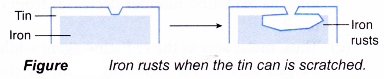
- In tin plating, an iron or steel sheet is coated with a very thin coating of tin. This is done by dipping the iron into molten tin or by electroplating an iron sheet using tin(IV) chloride as the electrolyte.
- Tin plating is usually used for making tin cans as tin is very expensive. After tin plating, the inside of the can is coated with a thin layer of plastic.
- The tin provides a protective oxide coating to the cans. The cans do not rust as long as the tin coating remains unbroken.
- However, as soon as the can is scratched, rusting will occur quickly. This is because iron is more electropositive than tin. Thus, food in dented or scratched tin cans should not be consumed.
2. Alloying
Stainless steel is a corrosion resisting alloy of iron.
- It contains carbon and a varying amount of chromium and nickel. The typical stainless steel contains about 18% chromium and 8% nickel.
- Thus, stainless steel is expensive and is mainly used for small objects such as cutlery and decorative items.
- The chromium and nickel provide a protective oxide coating which is firmly bonded to the iron and is not easily removed. Furthermore, the oxide coating is shiny, hence giving stainless steel an attractive, mirror-like finish.
3. Sacrificial protection
- In this method, iron is attached to a more electropositive metal which acts as the sacrificial metal.
- It is used for objects that are exposed to conditions that speed up rusting such as water and sea water. For example, bridge pillars and hulls of ship are usually attached to zinc blocks while underground pipelines are tied to bags of magnesium.
- Being more electropositive, the sacrificial metal would act as the anode whereby it is oxidised, protecting iron from rusting. Thus, the sacrificial metal is also known as the sacrificial anode.
- The sacrificial metal has to-be renewed from time to time.
Effect of other metals on rusting experiment
Aim: To investigate the effect of other metals on rusting.
Problem statment: How do different types of metals in contact with iron affect rusting?
Hypothesis: When a more electropositive metal is in contact with iron, the metal inhibits rusting. When a less electropositive metal is in contact with iron, the metal speeds up rusting.
Variables:
(a) Manipulated variable : Different metals in contact with iron
(b) Responding variable : Presence of blue colouration
(c) Controlled variables : Clean iron nails, medium in which iron nails are kept, temperature
Operational definition:
Blue colouration indicates rusting of iron.
Materials: Iron nails, magnesium ribbon, copper strip, zinc strip, tin strip, hot jelly solution containing a little
potassium hexacyanoferrate(III) solution and phenolphthalein indicator, sandpaper.
Apparatus: Test tubes, test tube rack.
Safety measure:
Potassium hexacyanoferrate(III) solution is poisonous. Thus, the hot jelly solution should be handled with care.
Procedure:
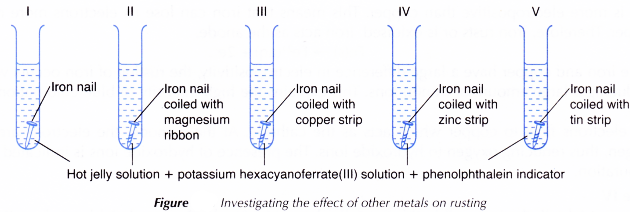
- All five iron nails, magnesium ribbon, strips of copper, zinc and tin are cleaned with sandpaper.
- Four iron nails are coiled tightly with magnesium ribbon, strips of copper, zinc and tin each.
- All five iron nails are placed in five separate test tubes as shown in Figure.
- The same amount of hot jelly solution containing potassium hexacyanoferrate(III) solution and phenolphthalein indicator is poured into the test tubes to completely cover ail the nails.
- The test tubes are kept in a test tube rack and left aside for a day. Any changes are observed.
Results:
| Test tube | Pair of metals | Intensity of dark blue colouration | Pink colouration | Inference regarding rusting |
| I | Fe only | Low | Present | The iron nail rusts a little. |
| II | Fe + Mg | None | Present | The iron nail does not rust. |
| III | Fe + Cu | Very high | Present | The iron nail rusts very quickly. |
| IV | Fe + Zn | None | Present | The iron nail does not rust. |
| V | Fe + Sn | High | Present | The iron nail rusts quickly. |
Discussion:
- During rusting, iron(II) ions are produced. These ions form dark blue colouration with potassium hexacyanoferrate(III). The more iron(II) ions are produced, the higher the intensity of the dark blue colouration.
- During the corrosion of a metal, the reduction of oxygen forms hydroxide ions, thus giving rise to basic condition. The hydroxide ions give pink colouration with phenolphthalein.
- The jelly is used to enable us see the blue and pink colourations clearly as diffusion occurs slowly in a solid state. Otherwise, the blue and pink colourations are mixed up and difficult to distinguish.
- Since pink colouration is found in all test tubes, it is inferred that corrosion of metal has taken place in each test tube. The metal that corrodes must either be the iron nail or the metal it is in contact with.
- Sometimes, the pink colouration is not clear as the hydroxide ions formed, immediately combine with the metal ions. Thus, not many free hydroxide ions are present in the jelly.
- Test tube I acts as a control. The iron nail rusts a little.
- Test tube II
(a) Magnesium is more electropositive than iron. This means that magnesium can lose its electrons more readily than iron. Therefore, magnesium is oxidised. Magnesium acts as the anode.
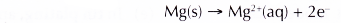
(b) The electrons flow to iron which acts as the cathode. At the cathode, the electrons are gained by
oxygen. Thus, oxygen undergoes reduction, producing hydroxide ions which give pink colouration with phenolphathalein.
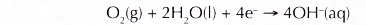
(c) The iron nail does not corrode. This explains the absence of blue colouration in this test tube. - Test tube III
(a) Iron is more electropositive than copper. This means that iron can lose its electrons more readily than copper. Therefore, iron rusts or is oxidised. Iron acts as the anode.
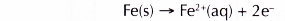
(b) Since iron and copper have a large difference in electropositivity, the rusting of iron occurs very quickly, producing a large amount of iron(II) ions. This explains the high intensity of blue colouration in this test tube.
(c) The electrons flow to copper which acts as the cathode. At the cathode, the electrons are gained by oxygen, thus reducing oxygen to hydroxide ions. The presence of hydroxide ions is indicated by the pink colouration. - Test tube IV
Similar to test tube II, the iron nail in this test tube does not corrode, thus no dark blue colouration is found.
Zinc acts as the anode and is oxidised as zinc is more electropositive than iron.
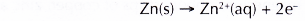
- Test tube V
Similar to test tube III, the iron nail acts as the anode and rusts. This is because iron is more electropositive than tin.
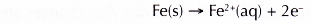
However, the rate of rusting in test tube V is lower than that in test tube III as the difference in electropositivity
between iron and tin is smaller than the difference in electropositivity between iron and copper.
Conclusions:
- Rusting is inhibited when iron is in contact with a more electropositive metal.
- Rusting is speeded up when iron is in contact with a less electropositive metal.