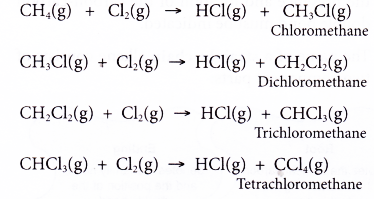
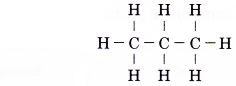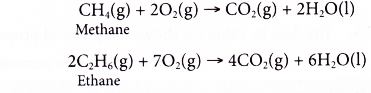What is alkenes and examples?
What is the general formula for an alkene?
Alkenes
- Alkenes form another family of hydrocarbons.
- The general formula for the alkene family is CnH2n, where n = 2, 3, 4, …
- Alkenes are unsaturated hydrocarbons. Every alkene has a carbon-carbon double bond.
- The carbon-carbon double bond is the functional group of the alkene family.
- A functional group is a group of atoms which determines the chemical properties of the organic molecule.
- Hence, the double bond determines the chemical reactions of alkenes.
How do you name alkenes?
Naming alkenes
- The naming of alkenes follows the same IUPAC rules as used for the naming of alkanes. However, the presence and location of the carbon-carbon double bond must be indicated.
- The name of a straight-chain alkene consists of two component parts:
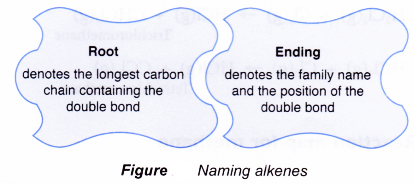
- Determining root name:
- Identify the longest continuous carbon chain that contains the double bond.
- Count the number of carbon atoms in this chain.
- Name it appropriately.
- Determining ending:
- Use the ending -ene to denote the alkene family.
- Number the carbon atoms in the longest chain. Begin numbering at the end nearer the double bond.
- Identify the location of the double bond by using the number of the first carbon atom in the double bond.
- Place the number in front of the ending -ene.
- For molecules containing more than three carbon atoms, the position of the double bond must be specified.
- Example shows the method used.
Naming alkenes example
Name the alkene molecule with the structure given below.
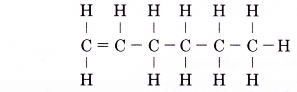
Solution:
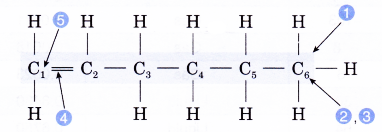
- Identify the longest carbon chain containing the double bond.
- Count the number of carbon atoms in the longest chain.
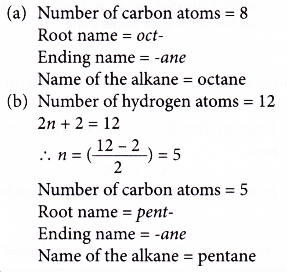
Number of carbon atoms = 6 - Give the root name.
Root name: hex- - Identify the molecule’s family.
Family: alkene
Ending: -ene - Number the carbon atoms from the end nearer to the double bond. Give the double bond the smaller number.
Position of the double bond: 1-ene - Complete the name for the alkene molecule.
Name of the alkene: hex-1-ene
People also ask
- What are carbon compounds?
- Chemical Properties of Carbon Compounds
- How are alkanes formed?
- What is an isomerism?
- What is alcohol and how is it made?
- How are carboxylic acids formed?
- How esters are formed?
- What are fats and oils?
- How palm oil is extracted?
- Order in Homologous Series
- What is the monomer of natural rubber?
- Which acid is used for coagulating rubber from latex?
- Classification of Hydrocarbons
- What is the homologous series of hydrocarbons?
- Properties and Uses of Ethanol
- Properties and Uses of Ethanoic Acid
What is the structure of an alkene?
Structural formulae of alkenes
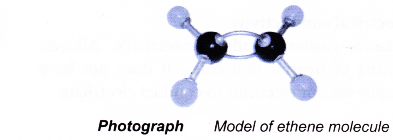
The first member of the alkene family is ethene, C2H4. A model of the ethene molecule is shown in Photograph.
The second member, propene, C3H6, is obtained by following the following steps.
- Step 1: Position the three carbon atoms in a row.

- Step 2: Join the first two carbon atoms with a double bond.

- Step 3: Join the remaining carbon atom with a single bond.

- Step 4: Place single bonds around each carbon atom. Ensure that each carbon atom has four bonds.
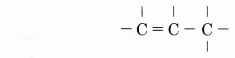
- Step 5: Attach a hydrogen atom to each of the single bonds of a carbon atom.
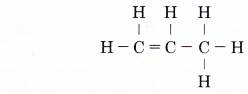
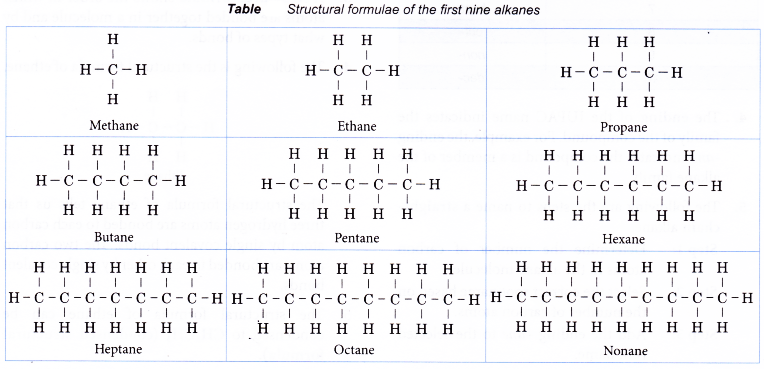
By using similar steps, the structural formulae of the other members of the alkene family can be obtained.
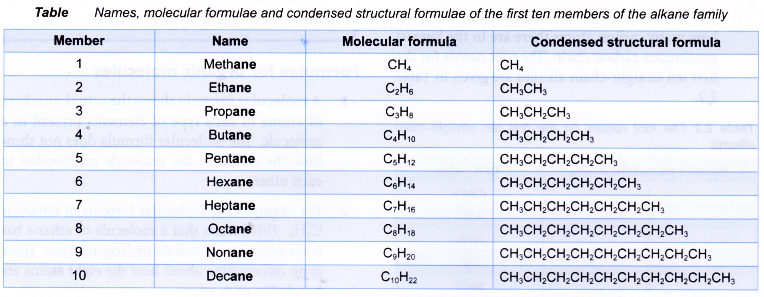
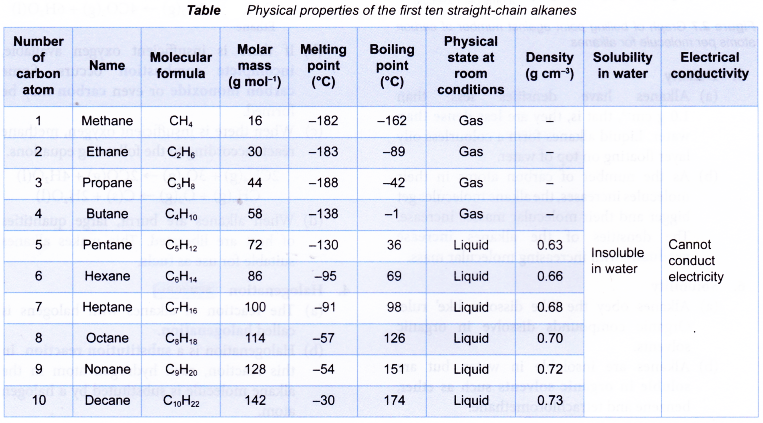
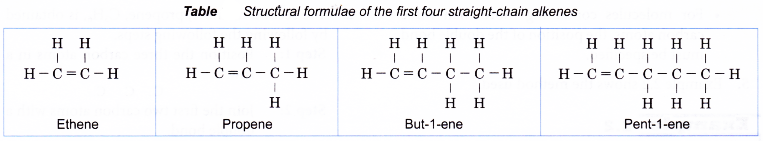
Table shows the names and molecular formulae of the first nine straight-chain alkenes.
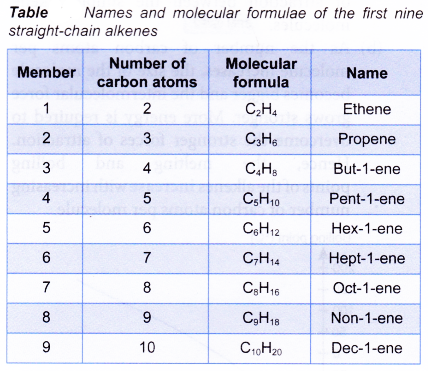
Table shows the structural formulae of the first four straight-chain alkenes.
What are the physical properties of alkenes?
Physical properties of alkenes
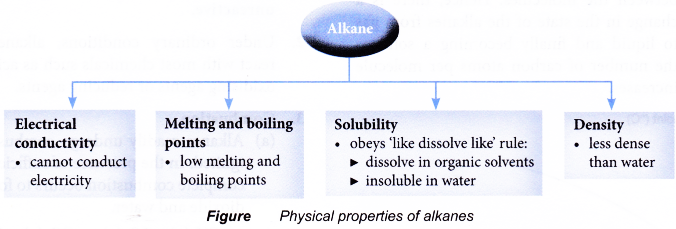
The physical properties of alkenes are similar to those of alkanes.
Table shows some physical properties of alkenes.
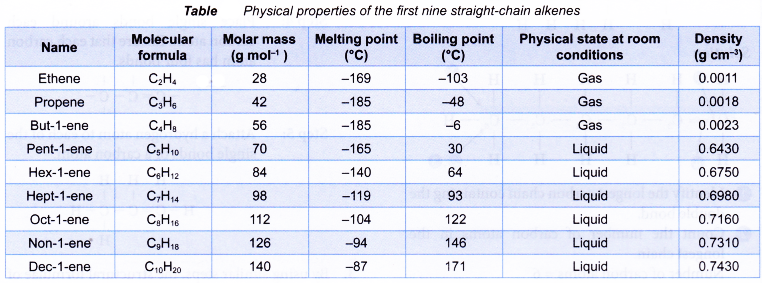
- Melting and boiling points
(a) Alkenes have low melting and boiling points. This is because less energy is required to overcome the weak intermolecular forces of attraction between the simple alkene molecules.
(b) As the number of carbon atoms per molecule increases, the size of the molecule becomes bigger and the intermolecular force grows stronger. More energy is required to overcome the stronger forces of attraction. Hence, the melting and boiling points of the alkenes increase with increasing number of carbon atoms per molecule.
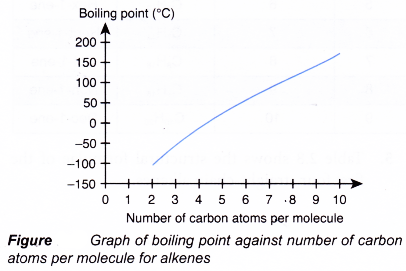
(c) Under room conditions, the first three alkenes, ethene, propene and butene, are gases. Those that contain five or more carbon atoms are liquids or solids. - Density
(a) Alkenes have low densities. Liquid and gaseous alkenes are less dense than water.
(b) Densities of the alkenes increase with increasing number of carbon atoms per molecule. - Solubility in water
(a) Alkenes do not dissolve in water but are soluble in organic solvents.
(b) When mixed with water, liquid alkenes form an oily layer floating on the surface of the water. - Electrical conductivity
Alkenes cannot conduct electricity. Alkenes consist of neutral molecules. It does not have mobile ions or electrons to conduct electricity.
What are the chemical properties of alkenes?
Alkenes are chemically more reactive than alkanes because carbon-carbon double bonds are more reactive than carbon-carbon single bonds. Almost all of the reactions of alkenes occur at the double bonds.
Combustion reaction
- Alkenes burn completely in excess oxygen to produce carbon dioxide and water. The complete combustion of ethene is shown by the following equation.
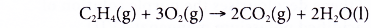
- However, alkenes burn with sootier flames as compared to alkanes. This is because alkenes have a higher percentage of carbon in their molecules than alkanes. The equations for the incomplete combustion of ethene show that carbon monoxide and soot (carbon) are produced.
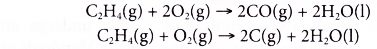
Addition reactions
- An addition reaction is a reaction in which atoms or groups of atoms are added to each carbon atom of a carbon-carbon double bond to form a single product.
- Addition of hydrogen
(a) Ethene reacts with hydrogen at 180°C in the presence of nickel or platinum catalyst to produce ethane. This process is called catalytic hydrogenation.
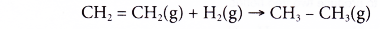
(b) Hydrogenation reaction is used to
(i) convert an unsaturated compound to a saturated compound.
(ii) prepare an alkane from an alkene. - Addition of halogens
(a) Halogenation is the addition of halogens to alkenes. Chlorine, bromine and iodine react readily with alkenes. No catalyst or ultraviolet light is needed.
(b) For example, when ethene is bubbled through a solution of bromine in water or tetrachloromethane in the dark, the red-brown bromine is decolourised and a colourless liquid, 1,2-dibromoethane, is formed.
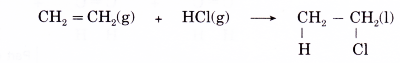
(c) Hence, bromine water is a reagent used as a test for the presence of a carbon-carbon double bond in organic molecules.
(d) Propene reacts with chlorine to form 1,2-dichloropropane. - Addition of hydrogen halides
(a) Hydrogen halides add readily across the double bond in alkenes. The reaction takes place rapidly at room temperature and requires no catalyst.
(b) The hydrogen halides that react with alkenes include hydrogen chloride, hydrogen bromide and hydrogen iodide.
(c) Ethene reacts with hydrogen chloride to form chloroethane according to the equation below.
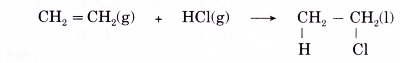
(d) Addition of hydrogen halides to propene can lead to the formation of two possible products. For example, in the reaction of hydrogen bromide with propene, two products might be expected, 1-bromopropane or 2-bromopropane, depending on whether the bromine atom from hydrogen bromide is attached to carbon 1 or carbon 2.
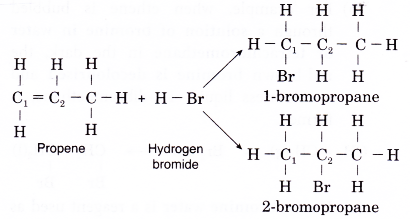
(e) In the above reaction, 2-bromopropane is the major product. A smaller amount of the alternative 1-bromopropane is also formed. - Addition of water
(a) Alkenes do not react with cold water under ordinary conditions. When a mixture of an alkene and steam is passed over a catalyst, a molecule of water is added to the carbon- carbon double bond to produce an alcohol.
(b) Addition of water to an alkene is called hydration. An example is the addition of water to ethene to produce ethanol.
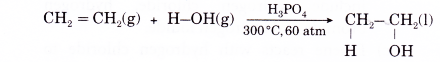
- Addition of hydroxyl groups
(a) When an alkene is treated with a dilute acidified potassium manganate(VII) solution, two hydroxyl groups are added to bromopropane the double bond. The product is a colourlessorganic compound called a diol.
(b) Ethene reacts with dilute acidified potassium manganate(VII) solution produce a product called ethane- 1,2-diol.
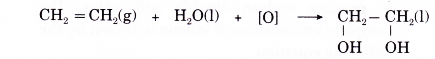
(c) Ethene decolourises the purple solution of acidified potassium manganate(VII).
(d) Acidified potassium manganate(VII) solution can be used as a reagent to test for the presence of a carbon-carbon double bond.
Polymerisation reaction
- Small alkene molecules readily undergo an addition reaction with one another. Hundreds or thousands of the alkene molecules link together to form long chains called polymers.
- This type of reaction to form a polymer from alkene monomers is called an addition polymerisation.
- The polymerisation of ethene is carried out by heating ethene at about 200°C and a pressure of 1200 atm in the presence of traces of oxygen. The reaction is summarised in Figure.
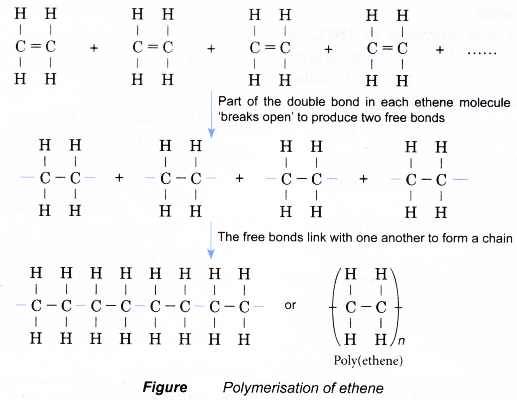
- The product poly(ethene), also known by its trade name polythene, is the most commonly used synthetic polymer. It is used for making film and sheeting for bags and wrappers and for making moulded articles such as toys, bottles and squeeze bottles.
Reaction map for ethene
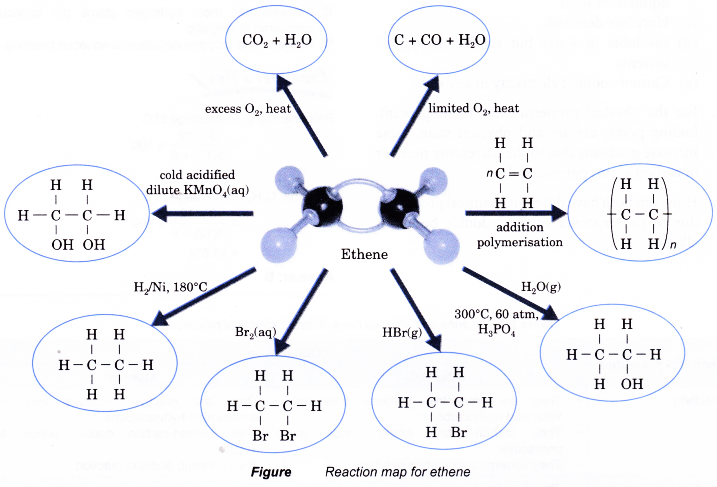
Properties of alkanes and alkenes
- Both alkanes and alkenes have almost similar physical properties as follows:
(a) Have low melting and boiling points
(b) Have physical state that changes from gas to liquid then solid
(c) Have low densities
(d) Insoluble in water but soluble in organic solvents
(e) Cannot conduct electricity in any state - For the physical properties like melting point, boiling point, density and physical state, these increase gradually due to the increasing number of carbon atoms per molecule.
- However, both have different chemical properties due to the existence of C=C double bonds in alkenes.
Table: Alkanes and alkenes have different chemical properties
| Chemical properties | Differences | |
| Alkanes | Alkenes | |
| Reactivity |
|
|
| Reaction with oxygen |
|
|
| Reaction with bromine |
|
|
| Reaction with acidified potassium manganate(VII) solution |
|
|
Experiment to compare the chemical properties of alkanes and alkenes
Aim: To compare the chemical properties of alkanes and alkenes.
Apparatus: Porcelain dishes, dropper, test tubes, Bunsen burner.
Materials: Hexane, hexene, bromine in 1,1,1 – trichloroethane, 0.1 mol dm-3 potassium manganate(VII) solution, dilute sulphuric acid, wooden splint, filter paper.
A. Reaction with oxygen.
Procedure:
- About 1 cm3 Of hexane and 1 cm3 of hexene are placed into two separate porcelain dishes.
- A lighted wooden splint is used to ignite the two liquids.
- The sootiness of the flame produced from the two burning hydrocarbons is observed and recorded.
- A piece of filter paper is held above each flame in both dishes as shown in Figure.
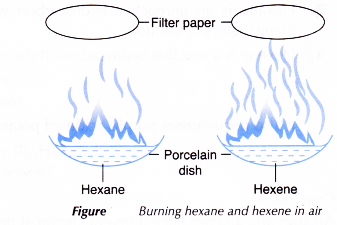
- The amount of soot collected on the two pieces of filter paper is noted and recorded.
B. Reaction with bromine
Procedure:
- About 2 cm3 of hexane is poured into a test tube.
- 2 to 3 drops of bromine in 1,1,1 -trichloroethane are added to the hexane. The mixture is shaken.
- Any change that occurs is noted and recorded.
- Steps 1 to 3 are repeated using hexene to replace hexane.
C. Reaction with acidified potassium manganate(VII) solution
Procedure:
- About 2 cm3 of dilute potassium manganate(VII) solution is poured into a test tube.
- About 2 cm3 of dilute sulphuric acid is added. The mixture is shaken.
- About 2 cm3 of hexane is added to the acidified potassium manganate(VII) solution. The mixture is shaken.
- Any change that occurs is noted and recorded.
- Steps 1 to 4 are repeated using hexene to replace hexane.
Observations:
| Reaction | Observation | |
| Hexane | Hexene | |
| Reaction with oxygen | Hexane burns with a yellow sooty flame. | Hexene burns with a yellow and very sooty flame. |
| Reaction with bromine | No visible change. | Hexene decolourises red-brown bromine. |
| Reaction with acidified potassium manganate(VII) solution | No visible change. | Hexene decolourises purple acidified potassium manganate(VII) solution. |
Discussion:
- Hexane, C6H14 is a member of the alkane family, whereas hexene, C6H12 belongs to the alkene family.
- Both hexane and hexene burn incompletely in air with a sooty flame. This is because both hydrocarbons have a high percentage by mass of carbon. Furthermore, air has only 21% oxygen.
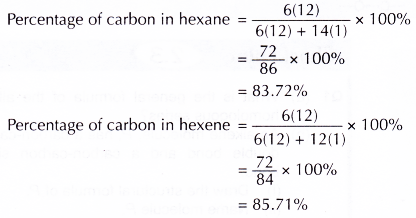
Hexene burns with a more sooty flame compared to hexane as its carbon content is much higher. - Hexane is an unreactive hydrocarbon which does not react with bromine water or acidified potassium manganate(VII) solution.
- Hexene is a reactive hydrocarbon. It decolourises red-brown bromine in an addition reaction.
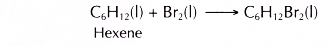
It also decolourises purple acidified potassium manganate(VI) to form a diol.
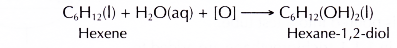
Conclusion:
Alkanes and alkenes have different chemical properties.