Chemical Properties of Carbon Compounds
Though we have millions of organic compounds, the reactions they undergo are limited. Some important reactions among them are discussed here.
They are:
1) Combustion reactions
2) Oxidation reactions
3) Addition reactions
4) Substitution reactions
1. Combustion reactions
Carbon, and its compounds burn in presence of oxygen or air to give CO2, heat and light.
The process of burning of carbon or carbon compound in excess of oxygen to give heat and light is called the combustion reaction. In the reactions carbon is in its maximum oxidation state of 4+ in the product.
Eg: 1) C + O2 → CO2 + Energy
2) 2C2H6 + 7O2 → 4CO2 + 6H2O + Energy
3) CH3CH2OH + 3O2 → CO2 + 3H2O + Energy
Generally, saturated hydrocarbons burn with a clear light blue flame, whereas unsaturated hydrocarbons burn with yellow flame with soot(carbon). If air is not sufficiently available during combustion, even saturated hydrocarbons give sooty flame. When Coal, Petroleum etc., burn in air they give oxides of sulphur and nitrogen in addition to CO2 and H2O which pollute the environment. When Coal or Charcoal is burnt sometimes they just glow red without flame. This is because to get a flame gaseous
fuels should burn.
Most of the aromatic compounds burn with sooty flame.
Because of the inlets of air getting closed, the fuel gases donot completely undergo combustion. Hence, it forms a sooty carbon form which gets coated over the vessels.A combustion reaction is generally defined as any reaction that sustains a flame. It usually involves burning with oxygen, though some exceptions
are there, combustion reaction is always exothermic, that is energy is liberated during combustion reaction.
Read More:
2. Oxidation reactions
Though combustion is generally oxidation reaction, all oxidation reactions are not combustion reactions. Oxidation reactions may be carried out using oxidizing agents. Oxidizing agents or Oxidants are substances that oxidize other substances. They themselves undergo reduction.
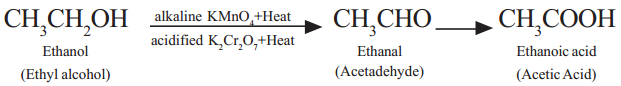
Eg: Alkaline Potassium permanganate or Acidified Potassium dichromate in solutions act as oxidizing agents and supplies oxygen to convert alcohols into carboxylic acids. Ethyl alcohol undergoes oxidation to form the product Acetaldehyde and finally Acetic acid.(see following equation).
3. Addition reactions
Unsaturated organic compounds that contain multiple bonds (=, ≡ bonds) like alkenes and alkynes undergo addition reactions to become saturated. During the reactions addition of the reagent takes place at the double bonded or triple bonded carbon atoms.
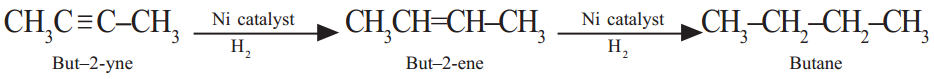
In the above reactions ‘Ni’ acts as ‘catalyst.’
Catalyst: A catalyst is a substance which regulates (increase/decrease) the rate of a given reaction without itself finally undergoing any chemical change.
These reactions are commonly used in the hydrogenation of vegetable oils using nickel as catalyst. Vegetable oils generally have long unsaturated carbon chains, while animal fats have saturated carbon chains.
Fats and oils are both of fatty acids. Oils are generally liquids at room temperature due to unsaturated fatty acids but fats are solids due to saturated fatty acids.
4. Substitution reactions
A reaction in which an atom or a group of atoms in a given compound is replaced by other atom or group of atoms is called a substitution reaction.
Alkanes, the saturated hydrocarbons are chemically least reactive.
Therefore they are also called paraffins (parum = little; affins = affinity, i.e., no affinity towards chemical changes). However they undergo some chemical changes under suitable conditions which are substitution reactions.
For example, methane (CH4) reacts with chlorine in the presence of sunlight. Hydrogen atoms of CH4 are replaced by chlorine atoms.

People also ask
- What are carbon compounds?
- How are alkanes formed?
- What is an alkene in chemistry?
- What is an isomerism?
- What is alcohol and how is it made?
- How are carboxylic acids formed?
- How esters are formed?
- What are fats and oils?
- How palm oil is extracted?
- Order in Homologous Series
- What is the monomer of natural rubber?
- Which acid is used for coagulating rubber from latex?
- Classification of Hydrocarbons
- What is the homologous series of hydrocarbons?
- Properties and Uses of Ethanol
- Properties and Uses of Ethanoic Acid