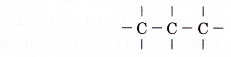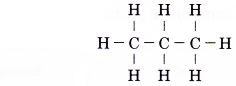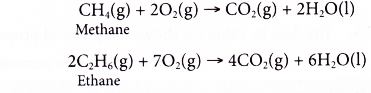What is an alkane in chemistry?
What is the general formula of the alkanes?
- The alkanes are a family of simple hydrocarbons with general formula CnH2n+2, where n= 1, 2, 3, …
- Alkanes are saturated hydrocarbons.
- Each carbon atom is bonded to four other atoms (carbon or hydrogen atoms) by single covalent bonds.
How do you name alkanes?
Naming the alkanes
- The current procedure for naming organic compounds is based on a set of rules laid down by the International Union of Pure and Applied Chemistry (IUPAC).
- The name of a straight-chain alkane consists of two component parts:
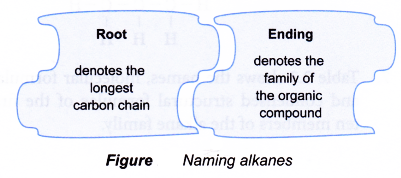
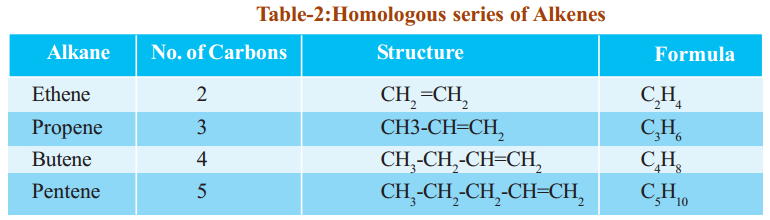
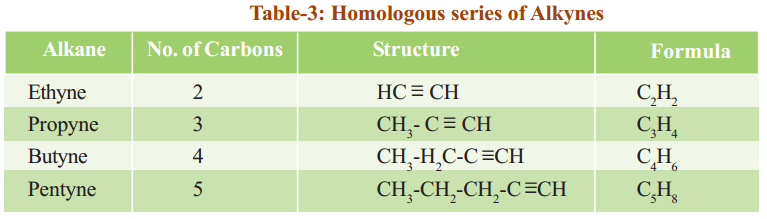
- The root part of the IUPAC name indicates how many carbon atoms there are in the longest continuous carbon chain. The root names for the first ten straight-chain alkanes are given in Table.
| Number of carbon atoms | Root name |
| 1 | meth- |
| 2 | eth- |
| 3 | prop- |
| 4 | but- |
| 5 | pent- |
| 6 | hex- |
| 7 | hept- |
| 8 | oct- |
| 9 | non- |
| 10 | dec- |
- The ending of the IUPAC name indicates the family of the compound. For example, the ending -ane indicates the compound is a member of the alkane family.
The following are the steps to name a straight-chain alkane.
- Step 1: Determine the number of carbon atoms in the alkane molecule.
- Step 2: Select the correct root name based on the number of carbon atoms.
- Step 3: Add the ending -ane to the selected root name.
Naming alkanes example
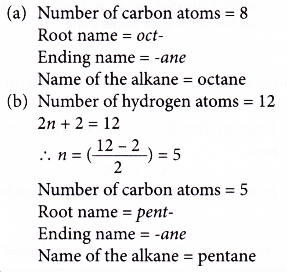
Name the following alkanes.
(a) C8H18
(b) CxH12
Solution:
People also ask
What is the molecular formula of alkane?
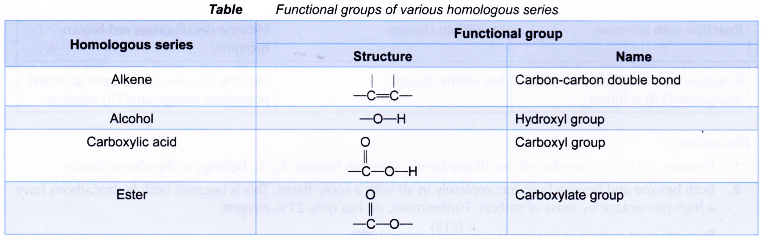
Formulae for organic molecules
- A molecular formula shows the actual numbers of atoms of each type of elements present in a molecule. The molecular formula does not show how the atoms in the molecule are bonded to each other.
- For example, the molecular formula of ethane is C2H6. This means that a molecule of ethane has two carbon atoms and six hydrogen atoms. There is no information about how the eight atoms are bonded to each other.
- A structural formula shows the order in which atoms are bonded together in a molecule and by what types of bonds.
- The following is the structural formula of ethane.
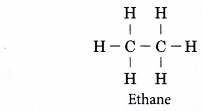
The structural formula of ethane tells us that three hydrogen atoms are bonded to each carbon atom by single covalent bonds. The two carbon atoms are bonded to each other by single covalent bonds. - The structural formula of ethane can be condensed to CH3CH3 (condensed structural formula).
The following shows how to draw the structural formula for propane, C3H8.
- Step 1: Position the three carbon atoms in a row.

- Step 2: Join the carbon atoms by single bonds.

- Step 3: Place four single bonds around each carbon atom.
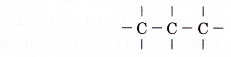
- Step 4: Attach a hydrogen atom to each ‘empty’ bond of a carbon atom.
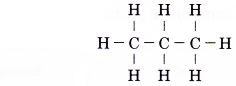
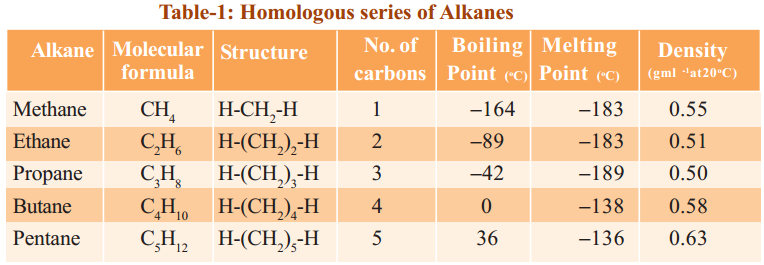
Table shows the names, molecular formulae and condensed structural formulae of the first ten members of the alkane family.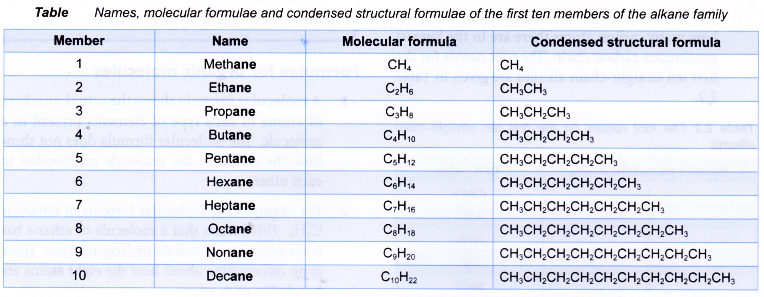
Table shows the structural formulae of the first nine members of the alkane family.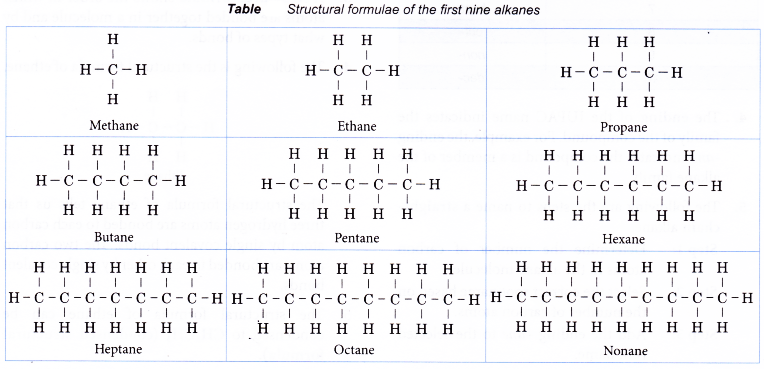
What are the physical and chemical properties of alkanes?
Physical properties of alkanes
Alkanes are covalent compounds which consist of simple molecules. These molecules are held together by weak inter molecular forces.
Hence, alkanes have physical properties that are typical of covalent compounds.
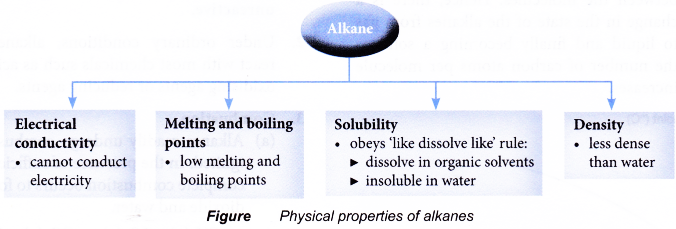
The data in Table shows the physical properties of the first ten straight-chain alkanes.
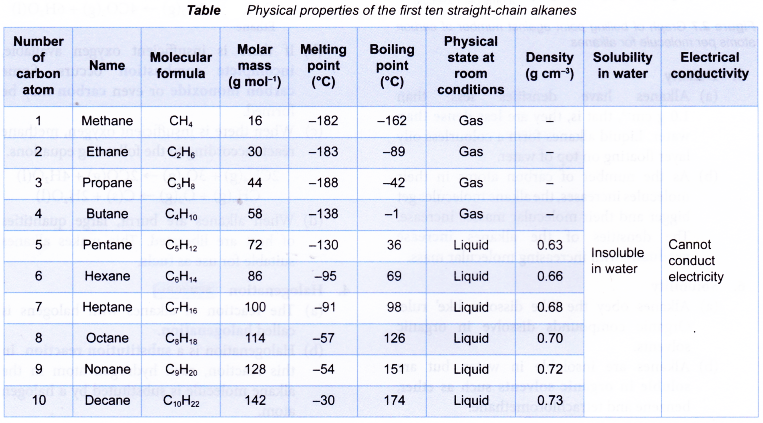
- In the alkane family, each member differs from a previous member only by having an additional CH2 unit. Hence, the members of the alkane family have closely related properties which vary in a systematic and predictable way. These properties show a gradual steady increase as the number of carbon atoms per molecule increases.
- Melting and boiling points
(a) Alkanes generally have low melting points and boiling points. This is because the molecules are held together by weak intermolecular forces which can be overcome by small amount of energy.
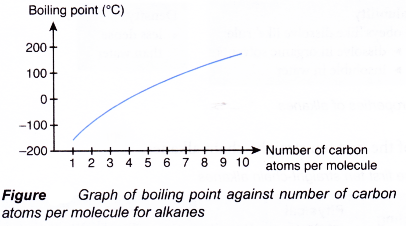
(b) However, there is a gradual increase in the melting and boiling points as the molecules become larger (Figure). More energy is needed to overcome the increasingly stronger intermolecular forces of attraction between the molecules. Hence, there is a change in the state of the alkanes from gas to liquid and finally becoming a solid as the number of carbon atoms per molecule increases.
- Density
(a) Alkanes have densities less than 1.0 g cm-3, that is, they are less dense than water. Liquid alkanes form a colourless oily layer floating on top of water.
(b) As the number of carbon atoms in their molecules increases, the alkane molecules get bigger and their molecular masses increase. The densities of the alkanes increase gradually with increasing molecular mass. - Solubility
(a) Alkanes obey the ‘like dissolve like’ rule. Organic compounds dissolve in organic solvents.
(b) Alkanes are insoluble in water, but are soluble in organic solvents such as ether, benzene and tetrachloromethane. - Electrical conductivity
(a) Alkanes cannot conduct electricity in any state.
(b) Alkanes are covalent molecules. The absence of mobile ions makes them unable to conduct electricity.
Chemical properties of alkanes
- Alkanes are saturated compounds with strong carbon-carbon (C – C) bonds and carbon- hydrogen (C – H) bonds. These bonds need a lot of energy to break. Hence, alkanes are generally unreactive.
- Under ordinary conditions, alkanes do not react with most chemicals such as acids, alkalis, oxidising agents or reducing agents.
- Combustion
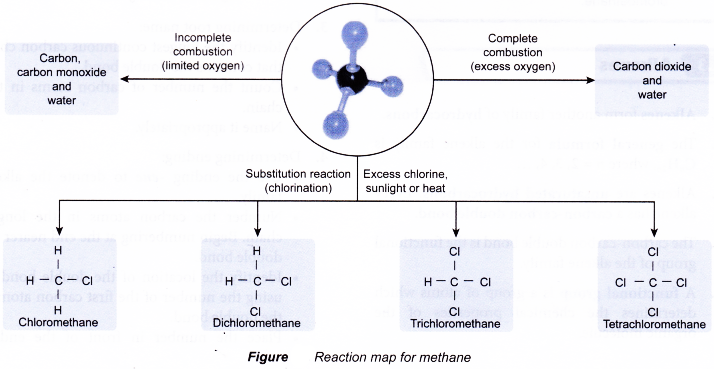
(a) Alkanes readily undergo combustion when ignited. In the presence of sufficient oxygen, complete combustion occurs to form carbon dioxide and water.
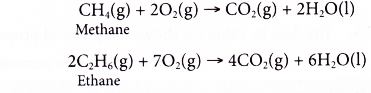
(b) If there is insufficient oxygen available, incomplete combustion occurs. Some carbon monoxide or even carbon may be formed.
(c) When there is insufficient oxygen, methane reacts according to the following equations.
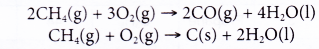
(d) When alkanes are burnt, large quantities of heat are liberated. This makes alkanes suitable for use as fuels. - Halogenation
(a) The reaction of alkanes with halogens is called halogenation.
(b) Halogenation is a substitution reaction. In this reaction, each hydrogen atom in the alkane molecule is substituted by a halogen atom.
(c) For example, when a mixture of methane and chlorine is exposed to ultraviolet light, a substitution reaction occurs. The hydrogen atoms in methane are replaced one by one by chorine atoms to produce four different products as shown in Photograph.
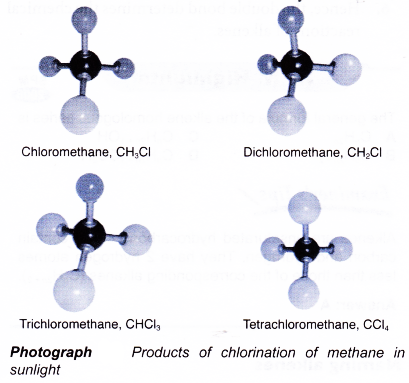
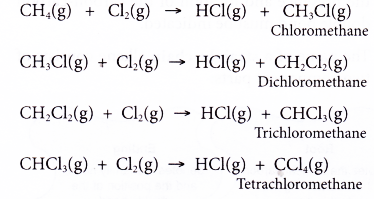
- The equations for the chlorination of methane are given below.
How methane can harm the environment?
- Methane, commonly known as natural gas, has many uses in everyday life.
- The uses of methane are as follows.
(a) As a fuel in a gas turbine and steam reboiler
(b) As a fuel for domestic heating and cooking
(c) As a feedstock for the production of hydrogen, methanol, acetic acid and acetic anhydride - Methane is formed as an end product of the anaerobic decay of plants. Certain organisms are able to decompose organic matter in the absence of oxygen. This explains the formation of methane gas in landfills and peat swamps. As methane is a combustible gas, it can cause fire in landfills and peat swamps.
- Methane is another important greenhouse gas. It is estimated that methane is 21 times more effective at trapping heat than carbon dioxide! The undesirable warming of the earth due to greenhouse effect will lead to serious consequences on the earth’s climate and to life on this planet.
Reaction map for methane