Is Carbon a metal, nonmetal or metalloid
The food you eat, the clothes you wear, the cosmetics you use, the fuels you use to run automobiles are all the compounds of carbon.
Carbon was discovered in prehistory and it was known to the ancients.
They used to manufacture charcoal by burning organic material.
Carbon is a non-metal. It belongs to the fourteenth group or IV A group in the modern periodical table. The elements of this group have four electrons in the valence shell.
Let us write the electronic configuration of Carbon (6C).
Atomic number of carbon is 6.
Electronic configuration of carbon (ground state) 6C: 1s2 2s2 2p2. To get the octet in its outer shell it has to gain four more electrons to form C4-. The electronegativity of carbon is only 2.5 and its nucleus has only six protons. Therefore it would be difficult for a nucleus with six protons to hold ten electrons. Hence, carbon cannot form C4- ions so easily.
If carbon loses four electrons from the outer shell, it has to form C4+ ions. This requires huge amount of energy which is not available normally.
Therefore C4+ formation also is a remote possibility. Carbon has to satisfy its tetravaiency by sharing electrons with other atoms. It has to form four covalent bonds either with its own atoms or atoms of other elements.
The possibility of bonds formation by a carbon atom is as:
a) i. Four single covalent bonds, with atoms of same element like hydrogen, Chlorine.
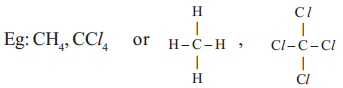
ii. Four single covalent bonds with atoms of different elements ;
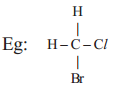
b) Carbon atoms may form one double bond and two single bonds
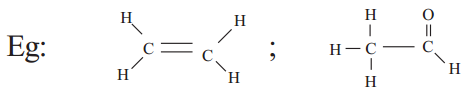
c) Carbon atom may form one single bond and a triple bond
Eg: H–C ≡ C–H or CH3–C ≡ N or carbon atoms may also form two double bonds as in CH2 = C = CH2.
Carbon atomic number is 6. Its mass number is 1.20. Its atomic mass is 12.011. Its melting point is 3550ºC and boiling point is 4830ºC. It occurs in free state as well as in combined state. 70% of our body is made up of carbon. It forms largest number of compounds. The earth crust contains only 0.02% of carbon.
Read More:
- Binding of Carbon with other Elements
- Homologous Series of Hydrocarbons
- Allotropes of Carbon
- Catenation in Carbon
- Classification of Hydrocarbons
- sp3 Hybridized Carbon atom
- Hybridization of the Carbon atoms in Acetylene
- Versatile Nature of Carbon
- What are the Characteristics of Compounds
- Chemical Properties of Carbon Compounds
- Nomenclature of Carbon Compounds
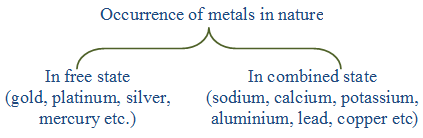 Metals usually occur in combination with nonmetallic elements. The native occurrence is comparatively rare.
Metals usually occur in combination with nonmetallic elements. The native occurrence is comparatively rare.