How metal is formed in the Earth
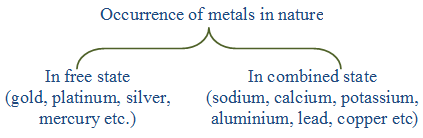
Metals occur in nature in free state or in combined state. A metal is said to occur native or free when it is found in nature in the metallic state. For example, gold may be found in nature as metal. This is because gold when left exposed to air practically does not undergo any change. It is not reacted upon by moisture, oxygen and carbon dioxide of the air. Thus, those metals which remain unaffected by moisture, oxygen and carbon dioxide of the air can occur native or free. In other words, the unreactive metals occur in nature in free state because of their low reactivity towards chemical reagents. Another example of an unreactive metal is silver.
The reactive metals, i.e., the metals which react with moisture, oxygen, carbon dioxide or other chemical reagents, are not found in nature in free state, but in combined state in the form of compounds.
 Metals usually occur in combination with nonmetallic elements. The native occurrence is comparatively rare.
Metals usually occur in combination with nonmetallic elements. The native occurrence is comparatively rare.
Minerals and Ores :
Metal-bearing substances, found in the earths crust, are called minerals. In other words, the solid compounds of metals occurring in nature are called minerals. For example, NaCl, KCl, CaCO3, MgCO3, ZnS, Cu2S, Fe2S3 etc., which are found in nature are minerals.
Some minerals and their occurrence in India are given below.
| Metal | Minerals | Places of occurrence in India |
| 1. Sodium 2. Magnesium 3. Calcium 4. Aluminium 5. Copper 6. Zinc 7. Manganese 8. Iron | Tincal, borax Dolomite, magnesite Gypsum Bauxite Chalcopyrites or copper pyrites, malachite Zincblende Pyrolusite Haematite | Ladakh (Kashmir) Tamil Nadu Rajasthan, Tamil Nadu, Jammu and Kashmir UP, Maharashtra,’MP, Orissa Jharkhand, Orissa, MP Rajasthan Maharashtra, Karnataka and Jharkhand Jharkhand and Karnataka |
Ores :
The minerals from which metals can be obtained on a commercial scale are called ores. In other words, the minerals from which metals can be extracted profitably are called ores. Both bauxite
(Al2O3.2H2O) and clay (Al2O3.2SiO2 . 2H2O) are minerals of aluminium. However, it is bauxite that is chiefly used to obtain aluminium commercially. So, bauxite, not clay, is an ore of aluminium. Thus:
(i) All ores are minerals, but all minerals are not ores.
(ii) An are is rich in the amount of the metal. The amount of foreign materials or impurities is low in an ore.
Different types of ores:
The different types of ores that are used in the extraction of metals are listed below.
1. Oxides : Copper, aluminium, zinc, tin, iron, etc., occur as oxides.
2. Sulphides : Silver, copper, zinc, mercury, lead, iron, etc., occur as sulphides.
3. Carbonates : Sodium, copper, calcium, magnesium, zinc, lead, iron, etc., occur as carbonates.
4. Sulphates : Sodium, calcium, magnesium, lead, etc., occur as sulphates.
5. Halides : Sodium, calcium, silver, etc., occur as halides.
6. Phosphates : Calcium occurs as phosphate.