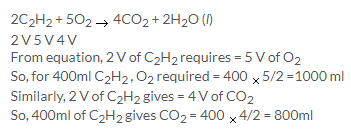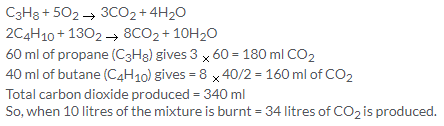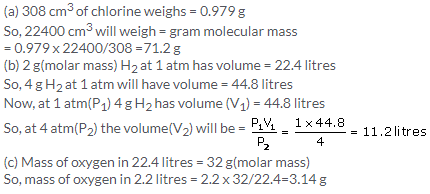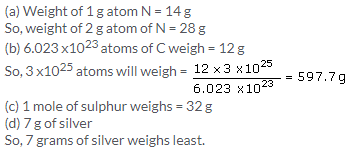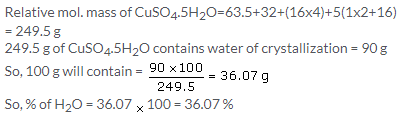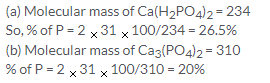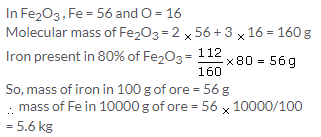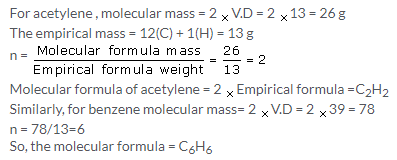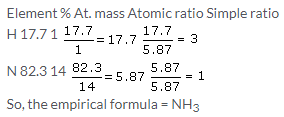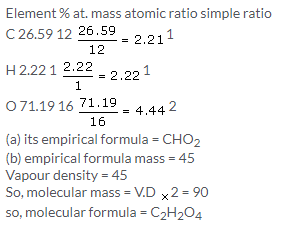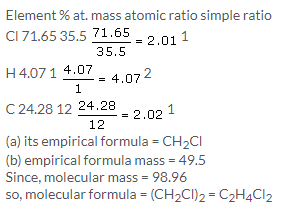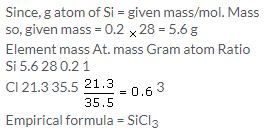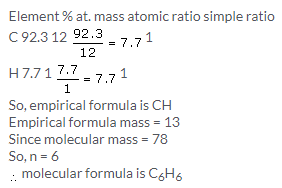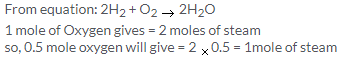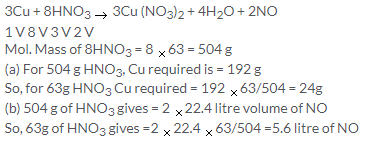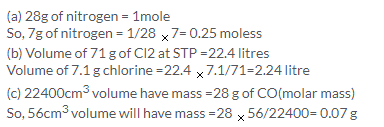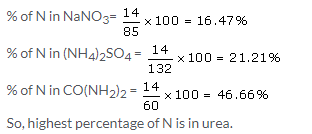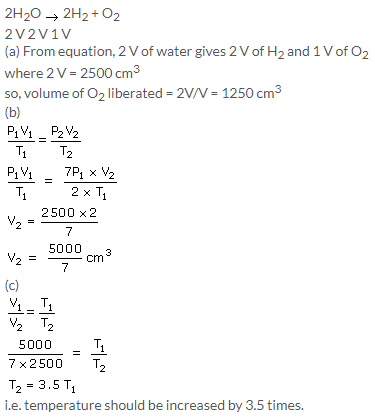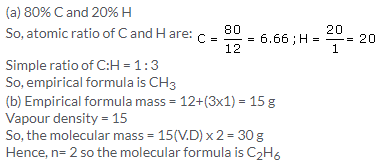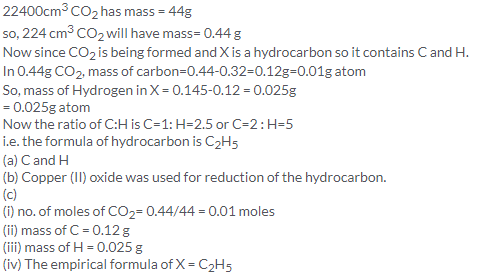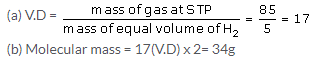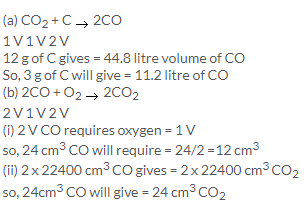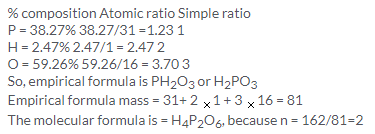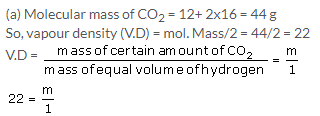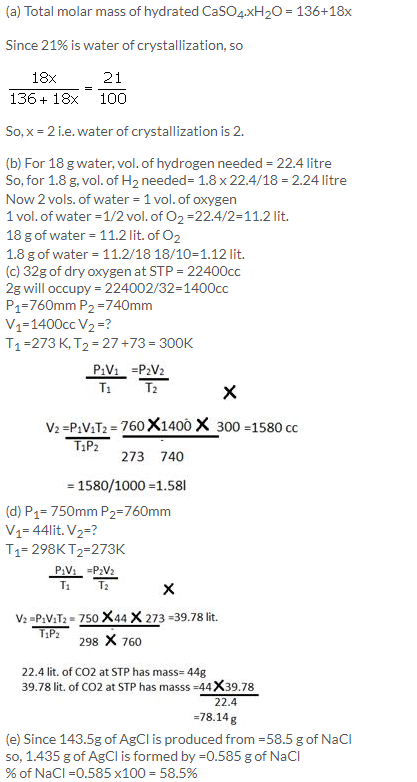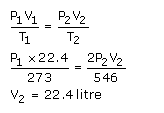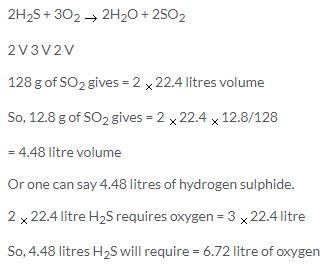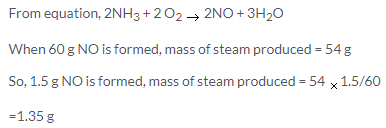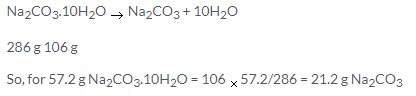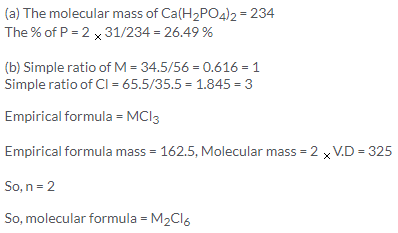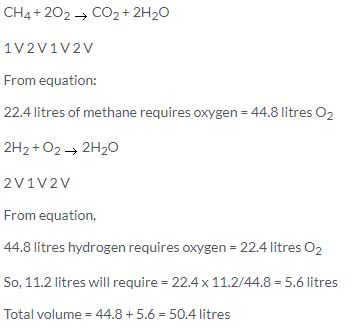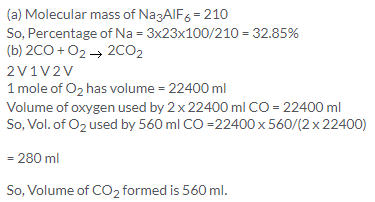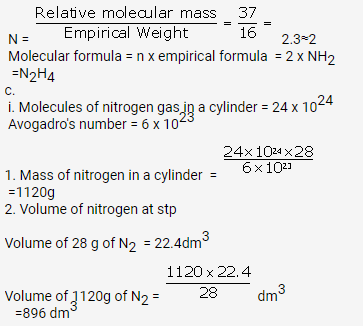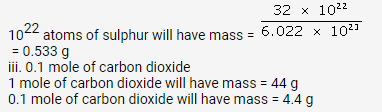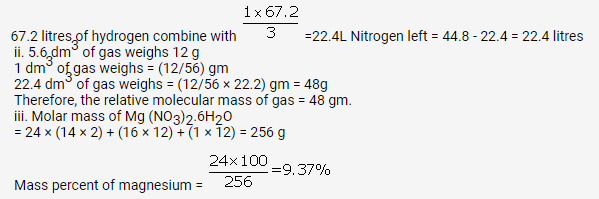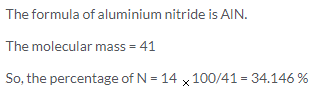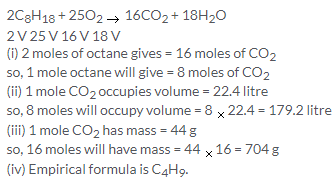Selina Concise Chemistry Class 10 ICSE Solutions Mole Concept and Stoichiometry
APlusTopper.com provides step by step solutions for Selina Concise ICSE Solutions for Class 10 Chemistry Chapter 5 Mole Concept and Stoichiometry. You can download the Selina Concise Chemistry ICSE Solutions for Class 10 with Free PDF download option. Selina Publishers Concise Chemistry for Class 10 ICSE Solutions all questions are solved and explained by expert teachers as per ICSE board guidelines.
Download Formulae Handbook For ICSE Class 9 and 10
ICSE SolutionsSelina ICSE Solutions
Selina ICSE Solutions for Class 10 Chemistry Chapter 5 Mole Concept and Stoichiometry
Exercise 5(A)
Solution 1.
(a) Gay-Lussac’s law states that when gases react, they do so in volumes which bear a simple ratio to one another, and to the volume of the gaseous product, provided that all the volumes are measured at the same temperature and pressure.
(b) Avogadro’s law states that equal volumes of all gases under similar conditions of temperature and pressure contain the same number of molecules.
Solution 2.
a) The number of atoms in a molecule of an element is called its atomicity. Atomicity of Hydrogen is 2, phosphorus is 4 and sulphur is 8.
b) N2means 1 molecule of nitrogen and 2N means two atoms of nitrogen.
N2 can exist independently but 2N cannot exist independently.
Solution 3.
(a) This is due to Avogadros Law which states Equal volumes of all gases under similar conditions of temperature and pressure contain the same number of molecules.
Now volume of hydrogen gas =volume of helium gas
n molecules of hydrogen =n molecules of helium gas
nH2=nHe
1 mol. of hydrogen has 2 atoms of hydrogen and I molecule of helium has 1 atom of helium
Therefore 2H=He
Therefore atoms in hydrogen is double the atoms of helium.
(b) For a given volume of gas under given temperature and pressure, a change in any one of the variable i.e., pressure or temperature changes the volume.
(c) Inflating a balloon seems violating Boyles law as volume is increasing with increase in pressure. Since the mass of gas is also increasing.
Solution 4.
2H2 + O2 → 2H2O
2 V 1V 2V
From the equation, 2V of hydrogen reacts with 1V of oxygen
so 200cm3 of Hydrogen reacts with = 200/2= 100 cm3
Hence, the unreacted oxygen is 150 – 100 = 50cm3 of oxygen.
Solution 5.

Solution 6.
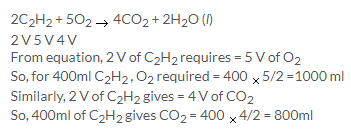
Solution 7.
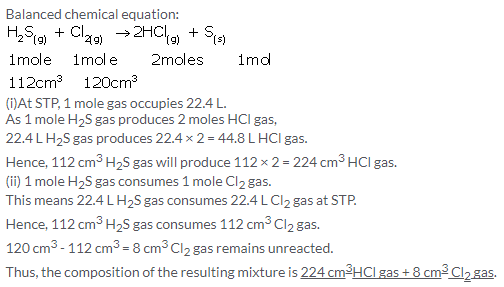
Solution 8.
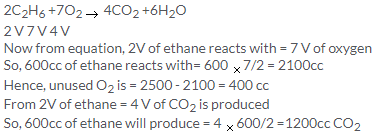
Solution 9.
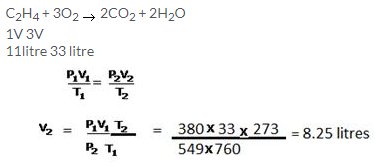
Solution 10.
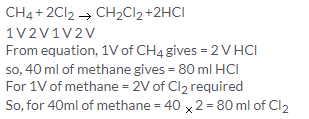
Solution 11.
C3H8 + 5O2 → 3CO2 + 4H2O
1 V 5 V 3 V
From equation, 5 V of O2 required = 1V of propane
so, 100 cm3 of O2 will require = 20 cm3 of propane
Solution 12.
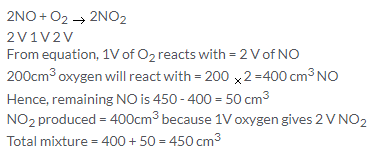
Solution 13.
2CO + O2 → 2CO2
2 V 1 V 2 V
2 V of CO requires = 1V of O2
so, 100 litres of CO requires = 50 litre of O2
Solution 14.
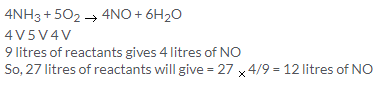
Solution 15.
H2 + Cl2 → 2HCl
1V 1V 2 V
Since 1 V hydrogen requires 1 V of oxygen and 4cm3 of H2 remained behind so the mixture had com”>16 cm3 hydrogen and 16 cm3 chlorine.
Therefore Resulting mixture is H2 =4cm3,HCl=32cm3
Solution 16.
CH4 + 2O2 → CO2 + 2H2O
1 V 2 V 1 V
2C2H2 + 5O2 → 4CO2 + 2H2O
2 V 5 V 4 V
From the equations, we can see that
1V CH4 requires oxygen = 2 V O2
So, 10cm3 CH4 will require =20 cm3 O2
Similarly 2 V C2H2 requires = 5 V O2
So, 10 cm3 C2H2 will require = 25 cm3 O2
Now, 20 V O2 will be present in 100 V air and 25 V O2 will be present in 125 V air ,so the volume of air required is 225cm3
Solution 17.
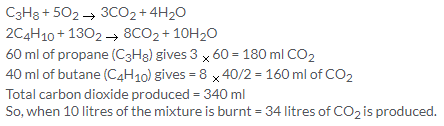
Solution 18.

Solution 19.
This experiment supports Gay lussac’s law of combining volumes.
Since the unchanged or remaining O2 is 58 cc so, used oxygen 106 – 58 = 48cc
According to Gay lussac’s law, the volumes of gases reacting should be in a simple ratio.
CH4 + 2O2 → CO2 + 2H2O
1 V 2 V
24 cc 48 cc
i.e. methane and oxygen react in a 1:2 ratio.
Solution 19.
According to Avogadro’s law, equal volumes of gases contain equal no. of molecules under similar conditions of temperature and pressure. This means more volume will contain more molecules and least volume will contain least molecules.
So,
(a) 5 litres of hydrogen has greatest no. of molecules with the maximum volume.
(b) 1 litre of SO2 contains the least number of molecules since it has the smallest volume.
Solution 20.
| Gas | Volume (in litres) | Number of molecules |
| Chlorine | 10 | x/2 |
| Nitrogen | 20 | x |
| Ammonia | 20 | X |
| Sulphur dioxide | 5 | x/4 |
Solution 21.

Exercise 5(B)
Solution 1.
a) This statement means one atom of chlorine is 35.5 times heavier than 1/12 time of the mass of an atom C-12.
b) The value of avogadro’s number is 6.023 × 1023
c) The molar volume of a gas at STP is 22.4 dm3 at STP
Solution 2.
(a) The vapour density is the ratio between the masses of equal volumes of gas and hydrogen under the conditions of standard temperature and pressure.
(b) Molar volume is the volume occupied by one mole of the gas at STP. It is equal to 22.4 dm3.
(c) The relative atomic mass of an element is the number of times one atom of the element is heavier than 1/12 times of the mass of an atom of carbon-12.
(d) The relative molecular mass of an compound is the number that represents how many times one moleculae of the substance is heavier than 1/12 of the mass of an atom of carbon-12.
(e) The number of atoms present in 12g (gram atomic mass) of C-12 isotope, i.e. 6.023 x1023 atoms.
(f) The quantity of the element which weighs equal to its gram atomic mass is called one gram atom of that element.
(g) Mole is the amount of a substance containing elementary particles like atoms, molecules or ions in 12 g of carbon-12.
Solution 3.
(a) Applications of Avogadro’s Law :
- It explains Gay-Lussac’s law.
- It determines atomicity of the gases.
- It determines the molecular formula of a gas.
- It determines the relation between molecular mass and vapour density.
- It gives the relationship between gram molecular mass and gram molecular volume.
(b) According to Avogadro’s law under the same conditions of temperature and pressure, equal volumes of different gases have the same number of molecules.
Since substances react in simple ratio by number of molecules, volumes of the gaseous reactants and products will also bear a simple ratio to one another.This what Gay Lussac’s Law says.
H2 + Cl2 → 2HCl
1V 1V 2V (By Gay-Lussacs law)
n molecules n molecules 2n molecules (By Avogadros law)
Solution 4.
(a) (2N)28 + (8H)8 + (Pt)195 + (6Cl)35.5 x 6 = 444
(b) KClO3 = (K)39 + (Cl)35.5 + (3O)48 = 122.5
(c) (Cu)63.5 + (S)32 + (4O)64 + (5H2O)5 x 18 = 249.5
(d) (2N)28 + (8H)8 + (S)32 + (4O)64 = 132
(e) (C)12 + (3H)3 + (C)12 + (2O)32 + (Na)23 = 82
(f) (C)12 + (H)1+ (3Cl)3 x 35.5 = 119.5
(g) (2N)28 + (8H)8 + (2Cr)2 x 51.9+ (7O)7 x 16 = 252
Solution 5.
(a) No. of molecules in 73 g HCl = 6.023 x1023 x 73/36.5(mol. mass of HCl)
= 12.04 x 1023
(b) Weight of 0.5 mole of O2 is = 32(mol. Mass of O2) x 0.5=16 g
(c) No. of molecules in 1.8 g H2O = 6.023 x 1023 x 1.8/18
= 6.023 x 1022
(d) No. of moles in 10g of CaCO3 = 10/100(mol. Mass CaCO3)
= 0.1 mole
(e) Weight of 0.2 mole H2 gas = 2(Mol. Mass) x 0.2 = 0.4 g
(f) No. of molecules in 3.2 g of SO2 = 6.023 x 1023 x 3.2/64
= 3.023 x 1022
Solution 6.
Molecular mass of H2O is 18, CO2 is 44, NH3 is 17 and CO is 28
So, the weight of 1 mole of CO2 is more than the other three.
Solution 7.
4g of NH3 having minimum molecular mass contain maximum molecules.
Solution 8.
a) No. of particles in s1 mole = 6.023 x 1023
So, particles in 0.1 mole = 6.023 x 10 23 x 0.1 = 6.023 x 1022
b) 1 mole of H2SO4 contains =2 x 6.023 x 1023
So, 0.1 mole of H2SO4 contains =2 x 6.023 x 1023 x0.1
= 1.2×1023 atoms of hydrogen
c) 111g CaCl2 contains = 6.023 x 1023 molecules
So, 1000 g contains = 5.42 x 1024 molecules
Solution 9.
(a) 1 mole of aluminium has mass = 27 g
So, 0.2 mole of aluminium has mass = 0.2 x 27 = 5.4 g
(b) 0.1 mole of HCl has mass = 0.1 x 36.5 (mass of 1 mole)
= 3.65 g
(c) 0.2 mole of H2O has mass = 0.2 x 18 = 3.6 g
(d) 0.1 mole of CO2 has mass = 0.1 x 44 = 4.4 g
Solution 10.
(a) 5.6 litres of gas at STP has mass = 12 g
So, 22.4 litre (molar volume) has mass =12 x 22.4/5.6
= 48g(molar mass)
(b) 1 mole of SO2 has volume = 22.4 litres
So, 2 moles will have = 22.4 x 2 = 44.8 litre
Solution 11.
(a) 1 mole of CO2 contains O2 = 32g
So, CO2 having 8 gm of O2 has no. of moles = 8/32 = 0.25 moles
(b) 16 g of methane has no. of moles = 1
So, 0.80 g of methane has no. of moles = 0.8/16 = 0.05 moles
Solution 12.
(a) 6.023 x 10 23 atoms of oxygen has mass = 16 g
So, 1 atom has mass = 16/6.023 x 1023 = 2.656 x 10-23 g
(b) 1 atom of Hydrogen has mass = 1/6.023 x 1023 = 1.666 x 10-24
(c) 1 molecule of NH3 has mass = 17/6.023 x1023 = 2.82 x 10-23 g
(d) 1 atom of silver has mass = 108/6.023 x 1023 =1.701 x 10-22
(e) 1 molecule of O2 has mass = 32/6.023 x 1023 = 5.314 x 10-23 g
(f) 0.25 gram atom of calcium has mass = 0.25 x 40 = 10g
Solution 13.
(a) 0.1 mole of CaCO3 has mass =100(molar mass) x 0.1=10 g
(b) 0.1 mole of Na2SO4.10H2O has mass = 322 x 0.1 = 32.2 g
(c) 0.1 mole of CaCl2 has mass = 111 x 0.1 = 11.1g
(d) 0.1 mole of Mg has mass = 24 x 0.1 = 2.4 g
Solution 14.
1molecule of Na2CO3.10H2O contains oxygen atoms = 13
So, 6.023 x1023 molecules (1mole) has atoms=13 x 6.023 x 1023
So, 0.1 mole will have atoms = 0.1 x 13 x 6.023 x 1023 =7.8 x 1023
Solution 15.
3.2 g of S has number of atoms = 6.023 x1023 x 3.2 /32
= 0.6023 x 1023
So, 0.6023 x 1023 atoms of Ca has mass=40 x0.6023×1023/6.023 x 1023
= 4g
Solution 16.
(a) No. of atoms = 52 x 6.023 x1023 = 3.131 x 1025
(b) 4 amu = 1 atom of He
so, 52 amu = 13 atoms of He
(c) 4 g of He has atoms = 6.023 x1023
So, 52 g will have = 6.023 x 1023 x 52/4 = 7.828 x1024 atoms
Solution 17.
Molecular mass of Na2CO3 = 106 g
106 g has 2 x 6.023 x1023 atoms of Na
So, 5.3g will have = 2 x 6.023 x1023x 5.3/106=6.022 x1022 atoms
Number of atoms of C = 6.023 x1023 x 5.3/106 = 3.01 x 1022 atoms
And atoms of O = 3 x 6.023 x 1023 x 5.3/106= 9.03 x1022 atoms
Solution 18.
(a) 60 g urea has mass of nitrogen(N2) = 28 g
So, 5000 g urea will have mass = 28 x 5000/60 = 2.33 kg
(b) 64 g has volume = 22.4 litre
So, 320 g will have volume = 22.4 x 320/64=112 litres
Solution 19.
(a) Vapour density of carbon dioxide is 22, it means that 1 molecule of carbon dioxide is 22 heavier than 1 molecule of hydrogen.
(b) Vapour density of Chlorine atom is 35.5.
Solution 20.
22400 cm3 of CO has mass = 28 g
So, 56 cm3 will have mass = 56 x 28/22400 = 0.07 g
Solution 21.
18 g of water has number of molecules = 6.023 x 1023
So, 0.09 g of water will have no. of molecules = 6.023 x 1023 x 0.09/18 = 3.01 x 1021 molecules
Solution 22.
(a) No. of moles in 256 g S8 = 1 mole
So, no. of moles in 5.12 g = 5.12/256 = 0.02 moles
(b) No. of molecules = 0.02 x 6.023 x 1023 = 1.2 x 1022 molecules
No. of atoms in 1 molecule of S = 8
So, no. of atoms in 1.2 x 1022 molecules = 1.2 x 1022 x 8
= 9.635x 1022 molecules
Solution 23.
Atomic mass of phosphorus P = 30.97 g
Hence, molar mass of P4 = 123.88 g
If phosphorus is considered as P4 molecules,
then 1 mole P4 ≡ 123.88 g
Therefore, 100 g of P4 = 0.807 g
Solution 24.
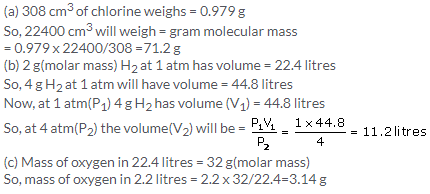
Solution 25.
No. of atoms in 12 g C = 6.023 x1023
So, no. of carbon atoms in 10-12 g = 10-12 x 6.023 x1023/12
= 5.019 x 1010 atoms
Solution 26.
Given:
P= 1140 mm Hg
Density = D = 2.4 g / L
T = 273 0C = 273+273 = 546 K
M = ?
We know that, at STP, the volume of one mole of any gas is 22.4 L
Hence we have to find out the volume of the unknown gas at STP.
First apply Charle’s law.
We have to find out the volume of one liter of unknown gas at standard temperature 273 K.
V1= 1 L T1 = 546 K
V2=? T2 = 273 K
V1/T1 = V2/ T2
V2 = (V1 x T2)/T1
= (1 L x 273 K)/546 K
= 0.5 L
We have found out the volume at standard temperature. Now we have to find out the volume at standard pressure.
Apply Boyle’s law.
P 1 = 1140 mm Hg V1 = 0.5 L
P2 = 760 mm Hg V2 = ?
P1 x V1 = P2 x V2
V2 = (P1 x V1)/P2
= (1140 mm Hg x 0.5 L)/760 mm Hg
= 0.75 L
Now, 22.4 L is the volume of 1 mole of any gas at STP, then 0.75 L is the volume of X moles at STP
X moles = 0.75 L / 22.4 L
= 0.0335 moles
The original mass is 2.4 g
n = m / M
0.0335 moles = 2.4 g / M
M = 2.4 g / 0.0335 moles
M= 71.6 g / mole
Hence, the gram molecular mass of the unknown gas is 71.6 g
Solution 27.
1000 g of sugar costs = Rs. 40
So, 342g(molar mass) of sugar will cost=342×40/1000=Rs. 13.68
Solution 28.
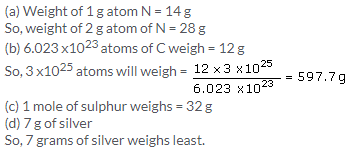
Solution 29.
40 g of NaOH contains 6.023 x 1023 molecules
So, 4 g of NaOH contains = 6.02 x1023 x 4/40
= 6.02 x1022 molecules
Solution 30.
The number of molecules in 18 g of ammonia= 6.02 x1023
So, no. of molecules in 4.25 g of ammonia = 6.02 x 1023 x 4.25/18
= 1.5 x 1023
Solution 31.
(a) One mole of chlorine contains 6.023 x 1023 atoms of chlorine.
(b) Under similar conditions of temperature and pressure, two volumes of hydrogen combined with one volume of oxygen will give two volumes of water vapour.
(c) Relative atomic mass of an element is the number of times one atom of an element is heavier than 1/12 the mass of an atom of carbon-12.
(d) Under similar conditions of temperature and pressure, equal volumes of all gases contain the same number of molecules.
Exercise 5(C)
Solution 1.
Information conveyed by H2O
- That H2O contains 2 volumes of hydrogen and 1 volume of oxygen.
- That ratio by weight of hydrogen and oxygen is 1:8.
- That molecular weight of H2O is 18g.
Solution 2.
The empirical formula is the simplest formula, which gives the simplest ratio in whole numbers of atoms of different elements present in one molecule of the compound.
The molecular formula of a compound denotes the actual number of atoms of different elements present in one molecule of a compound.
Solution 3.
(a) CH (b) CH2O (c) CH (d) CH2O
Solution 4.
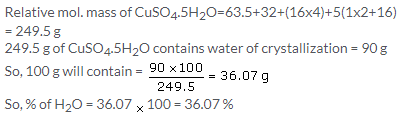
Solution 5.
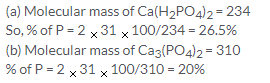
Solution 6.
Molecular mass of KClO3 = 122.5 g
% of K = 39 /122.5 = 31.8%
% of Cl = 35.5/122.5 = 28.98%
% of O = 3 x 16/122.5 = 39.18%
Solution 7.
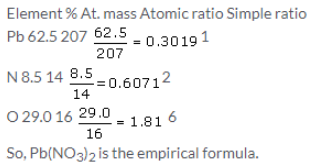
Solution 8.
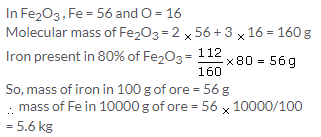
Solution 9.
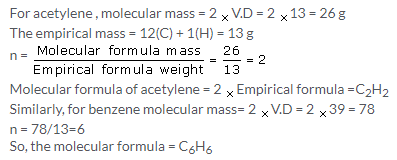
Solution 10.
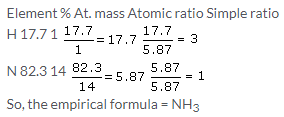
Solution 11.
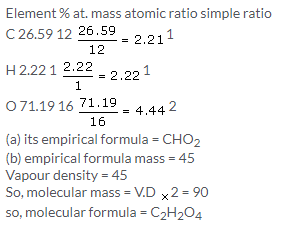
Solution 12.
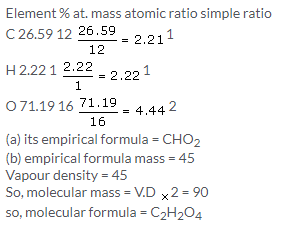
Solution 13.
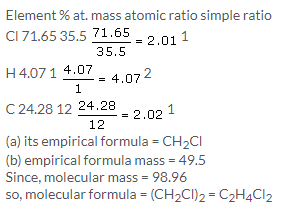
Solution 14.
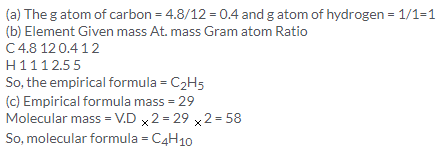
Solution 15.
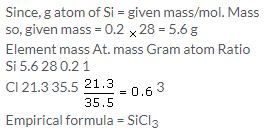
Solution 16.
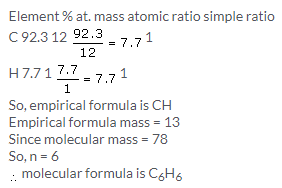
Solution 17.
(a) G atoms of magnesium = 18/24 = 0.75 or g- atom of Mg
(b) G atoms of nitrogen = 7/14 = 0.5 or 1/2 g- atoms of N
(c) Ratio of gram-atoms of N and Mg = 1:1.5 or 2:3
So, the formula is Mg3 N2
Solution 18.
Barium chloride = BaCl2.x H2O
Ba + 2Cl + x[H2 + O]
= 137+ 235.5 + x [2+16]
= [208 + 18x] contains water = 14.8% water in BaCl2.x H2O
= [208 + 18 x] 14.8/100 = 18x
= [104 + 9x] 2148=18000x
= [104+9x] 37=250x
= 3848 + 333x =2250x
1917x =3848
x = 2molecules of water
Solution 19.
Molar mass of urea; CON2H4 = 60 g
So, % of Nitrogen = 28 × 100/60 = 46.66%
Solution 20.
Element % At. mass Atomic ratio Simple ratio
C 42.1 12 3.5 1
H 6.48 1 6.48 2
O 51.42 16 3.2 1
The empirical formula is CH2O
Since the compound has 12 atoms of carbon, so the formula is
C12 H24 O12.
Solution 21.
(a) Now since the empirical formula is equal to vapour density and we know that vapour density is half of the molecular mass i.e. we have n=2 so, molecular formula is A2B4.
(b) Since molecular mass is 2 times the vapour density, so Mol. Mass = 2 V.D
Empirical formula weight = V.D/3
So, n = molecular mass/ Empirical formula weight = 6
Hence, the molecular formula is A6B6
Solution 22.
Atomic ratio of N = 87.5/14 = 6.25
Atomic ratio of H= 12.5/1 = 12.5
This gives us the simplest ratio as 1:2
So, the molecular formula is NH2
Solution 23.
Element % at. mass atomic ratio simple ratio
Zn 22.65 65 0.348 1
H 4.88 1 4.88 14
S 11.15 32 0.348 1
O 61.32 16 3.83 11
Empirical formula of the given compound =ZnSH14O11
Empiricala formula mass = 65.37+32+141+11+16=287.37
Molecular mass = 287
n = Molecular mass/Empirical formula mass = 287/287=1
Molecular formula = ZnSO11H14
= ZnSO4.7H2O
Exercise 5(D)
Solution 1.

Solution 2.
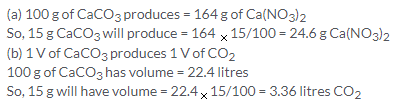
Solution 3.
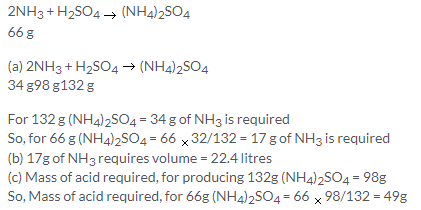
Solution 4.
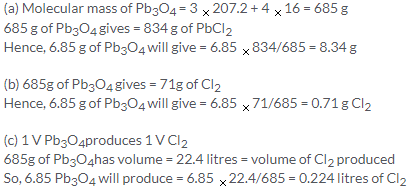
Solution 5.
Molecular mass of KNO3 = 101 g
63 g of HNO3 is formed by = 101 g of KNO3
So, 126000 g of HNO3 is formed by = 126000 x 101/63 = 202 kg
Similarly,126 g of HNO3 is formed by 170 kg of NaNO3
So, smaller mass of NaNO3 is required.
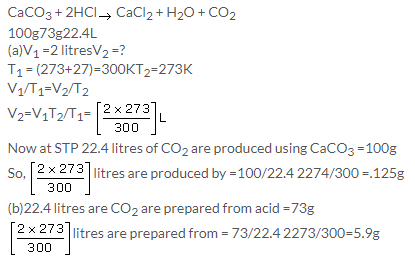
Solution 6.
Solution 7.
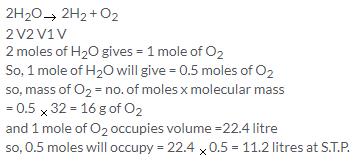
Solution 8.
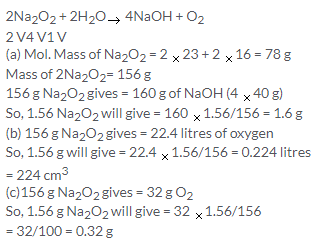
Solution 9.
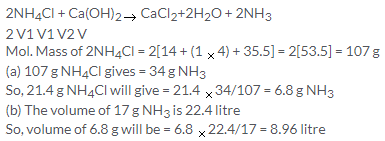
Solution 10.
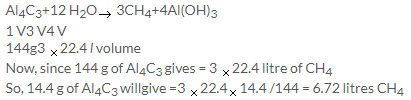
Solution 11.

Solution 12.

Miscellaneous Exercise
Solution 1.
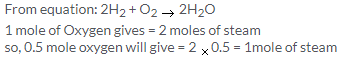
Solution 2.
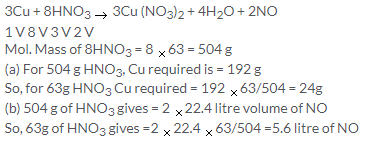
Solution 3.
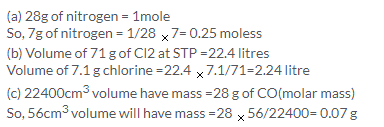
Solution 4.
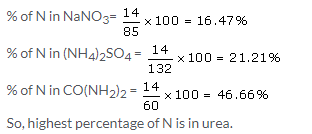
Solution 5.
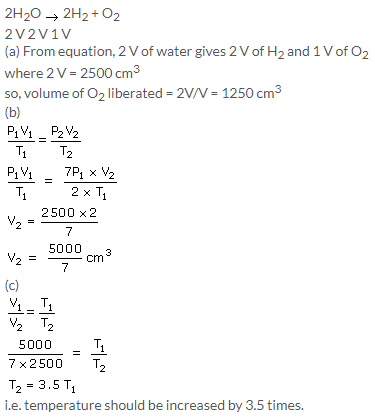
Solution 6.
Molecular mass of urea=12 + 16+2(14+2) =60g
60g of urea contains nitrogen =28g
So, in 50g of urea, nitrogen present =23.33 g
50 kg of urea contains nitrogen=23.33kg
Solution 7.
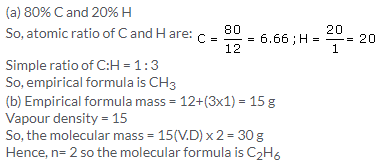
Solution 8.
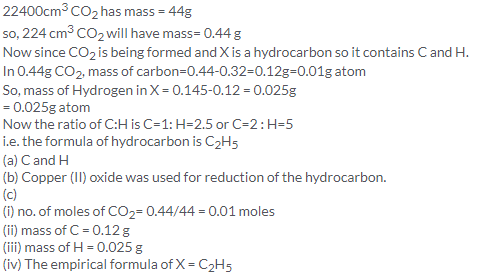
Solution 9.
Mass of X in the given compound =24g
Mass of oxygen in the given compound =64g
So total mass of the compound =24+64=88g
% of X in the compound = 24/88 100 = 27.3%
% of oxygen in the compound=64/88 100 =72.7%
Element % At. Mass Atomic ratio Simplest ratio
X 27.3 12 27.3/12=2.27 1
O 72.7 16 72.2/16=4.54 2
So simplest formula = XO2
Solution 10.
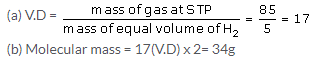
Solution 11.
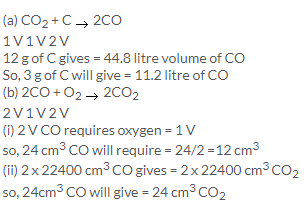
Solution 12.

Solution 13.
(a) Number of molecules in 100cm3 of oxygen=Y
According to Avogadros law, Equal volumes of all gases under similar conditions of temperature and pressure contain equal number of molecules.Therefore ,number of molecules in 100 cm3 of nitrogen under the same conditions of temperature and pressure = Y
So, number of molecules in 50 cm3 of nitrogen under the same conditions of temperature and pressure =Y/100 50=Y/2
(b) (i) Empirical formula is the formula which tells about the simplest ratio of combining capacity of elements present in a compound.
(ii) The empirical formula is CH3
(iii) The empirical formula mass for CH2O = 30
V.D = 30
Molecular formula mass = V.D 2 = 60
Hence, n =mol. Formula mass/empirical formula mass= 2
So, molecular formula = (CH2O)2 = C2H4O2
Solution 14.
The relative atomic mass of Cl = (35 x 3 + 1 x 37)/4=35.5 amu
Solution 15.
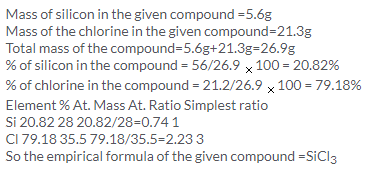
Solution 16.
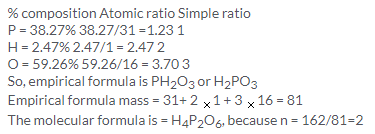
Solution 17.
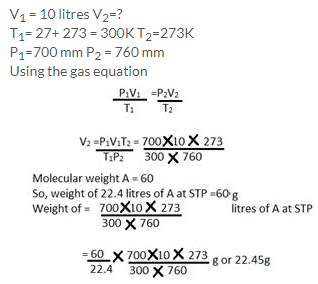
Solution 18.
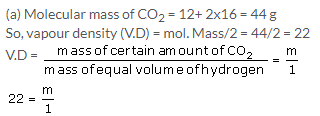
So, mass of CO2 = 22 kg
(b) According to Avogadros law ,equal volumes of all gases under similar conditions of temperature and pressure contain equal number of molecules.
So, number of molecules of carbon dioxide in the cylinder =number of molecules of hydrogen in the cylinder=X
Solution 19.
(a) The volume occupied by 1 mole of chlorine = 22.4 litre
(b) Since PV=constant so, if pressure is doubled; the volume will become half i.e. 11.2 litres.
(c) V1/V2 = T1/T2
22.4/V2 =273/546
V2 = 44.8 litres
(d) Mass of 1 mole Cl2 gas =35.5 x 2 =71 g
Solution 20.
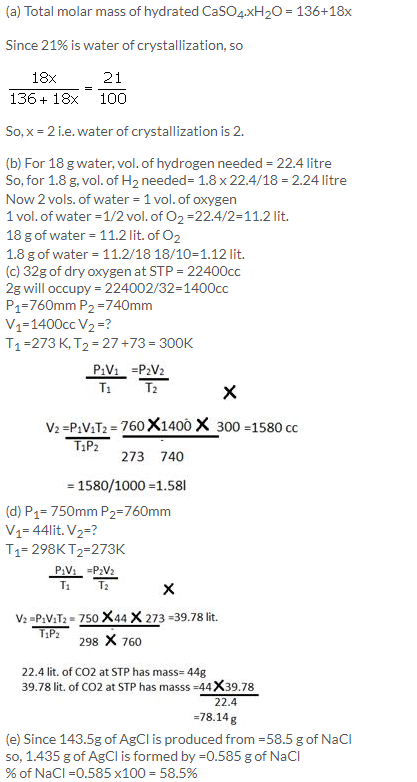
Solution 21.
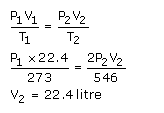
Solution 22.
(a) The molecular mass of (Mg(NO3)2.6H2O = 256.4 g
% of Oxygen = 12 x 16/256
= 75%
(b) The molecular mass of boron in Na2B4O7.10H2O = 382 g
% of B = 4 x 11/382 = 11.5%
Solution 23.

Solution 24.
(a) 252 g of solid ammonium dichromate decomposes to give 152 g of solid chromium oxide, so the loss in mass in terms of solid formed = 100 g
Now, if 63 g ammonium dichromate is decomposed, the loss in mass would be = 100 x 63/252 = 25 g
(b) If 252 g of ammonium dichromate produces Cr2O3 = 152 g
So, 63 g ammonium dichromate will produce = 63 x 152/252
= 38 g
Solution 25.
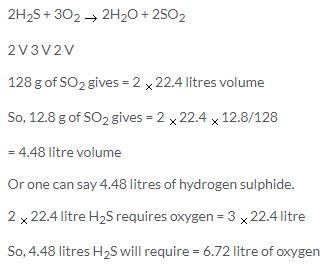
Solution 26.
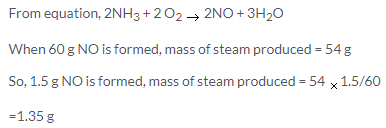
Solution 27.

Solution 28.

Since the pressure (760mm) remains constant , but the temperature (273+273)=546 is double, the volume of the steam also gets doubled
So,Volume of steam produced at 760mm Hg and 2730C = 4.48 × 2 = 8.96litre
Solution 29.

Solution 30.
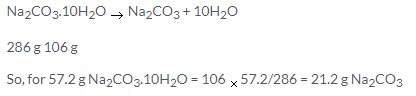
Solution 31.
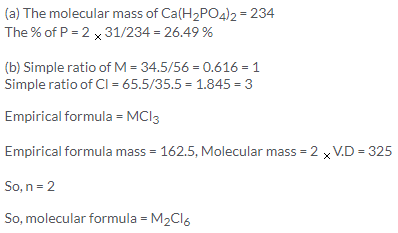
Solution 32.
V1/V2 = n1/n2
So, no. of moles of Cl = x/2 (since V is directly proportional to n)
No. of moles of NH3 = x
No. of moles of SO2 = x/4
This is because of Avogadros law which states Equal volumes of all gases, under similar conditions of temperature and pressure, contain equal number of molecules.
So, 20 litre nitrogen contains x molecules
So, 10 litre of chlorine will contain = x × 10/20=x/2 mols.
And 20 litre of ammonia will also contain =x molecules
And 5 litre of sulphur dioxide will contain = x × 5/20 = x/4 mols.
Solution 33.

Solution 34.
(a) Volume of O2 = V
Since O2 and N2 have same no. of molecules = x
so, the volume of N2 = V
(b) 3x molecules means 3V volume of CO
(c) 32 g oxygen is contained in = 44 g of CO2
So, 8 g oxygen is contained in = 44 x 8/32 = 11 g
(d) Avogadro’s law is used in the above questions.
Solution 35.
(a) 444 g is the molecular formula of (NH4)2 PtCl6
% of Pt = (195/444) x 100 = 43.91% or 44%
(b) simple ratio of Na = 42.1/23 = 1.83 = 3
simple ratio of P = 18.9/31 = 0.609 = 1
simple ratio of O = 39/16 = 2.43 = 4
So, the empirical formula is Na3PO4
Solution 36.
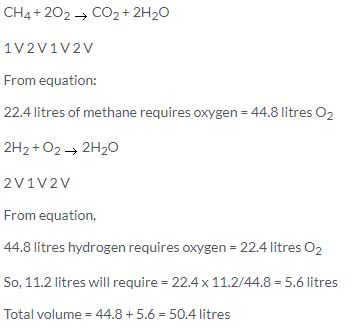
Solution 37.
According to Avogadros law:
Equal volumes of all gases, under similar conditions of temperature and pressure ,contain equal number of molecules.
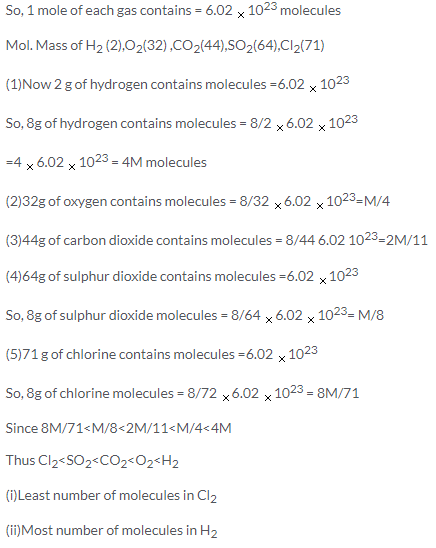
Solution 38.
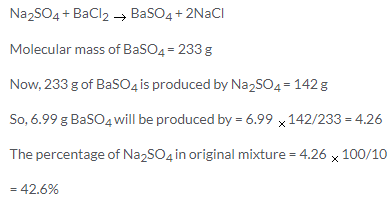
Solution 39.
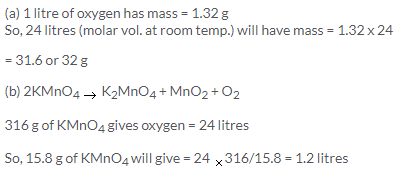
Solution 40.
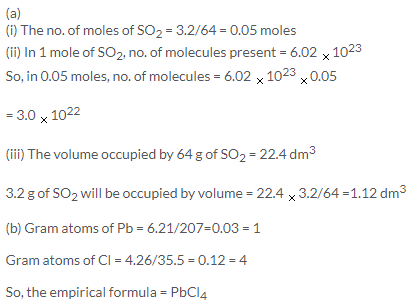
Solution 41.
(i) D contains the maximum number of molecules because volume is directly proportional to the number of molecules.
(ii) The volume will become double because volume is directly proportional to the no. of molecules at constant temperature and pressure.
V1/V2 = n1/n2
V1/V2 = n1/2n1
So, V2 = 2V1
(iii) Gay lussac’s law of combining volume is being observed.
(iv) The volume of D = 5.6 4 = 22.4 dm3, so the number of molecules = 6 x 1023 because according to mole concept 22.4 litre volume at STP has = 6 x 10 23 molecules
(v) No. of moles of D = 1 because volume is 22.4 litre
so, mass of N2O = 1 44 = 44 g
Solution 42.
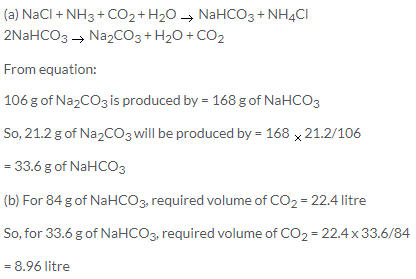
Solution 43.

Solution 44.
(a) Element % Atomic mass Atomic ratio Simple ratio
K 47.9 39 1.22 2
Be 5.5 9 0.6 1
F 46.6 19 2.45 4
so, empirical formula is K2BeF4
(b) 3CuO + 2NH3 → 3Cu + 3H2O + N2
3 V 2 V 3 V 1V
3 x 80 g of CuO reacts with = 2 x 22.4 litre of NH3
so, 120 g of CuO will react with = 2x 22.4 x 120/80 x 3
= 22.4 litres
Solution 45.
(a) The molecular mass of ethylene(C2H4) is 28 g
No. of moles = 1.4/28 = 0.05 moles
No. of molecules = 6.023 x1023 x 0.05 = 3 x 1022 molecules
Volume = 22.4 x 0.05 = 1.12 litres
(b) Molecular mass = 2 X V.D
S0, V.D = 28/2 = 14
Solution 46.
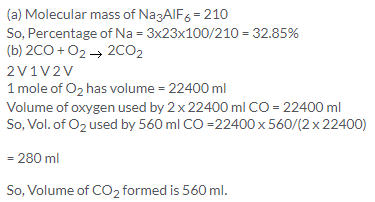
Solution 47.
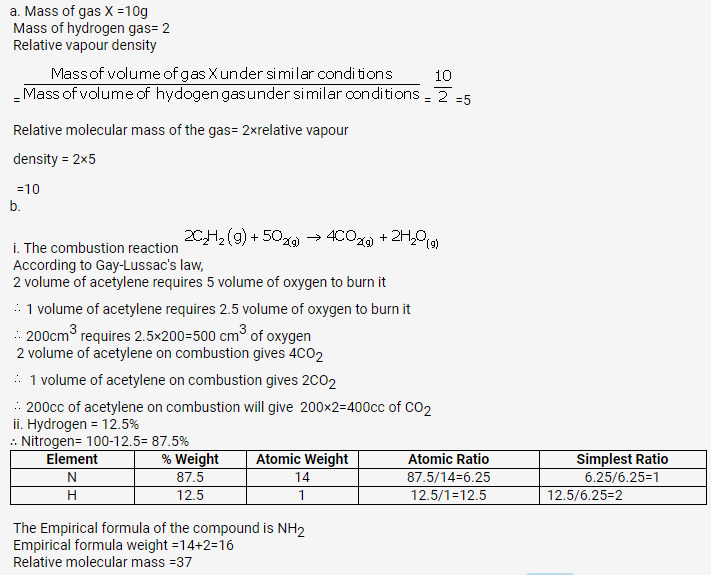
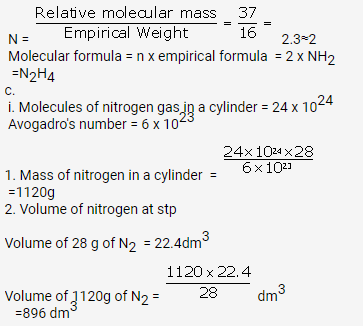
Solution 48.


Solution 49.
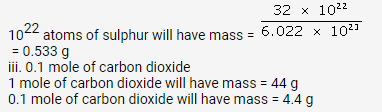
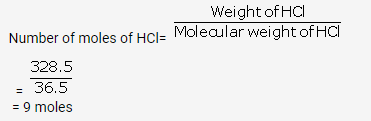
Solution 50.

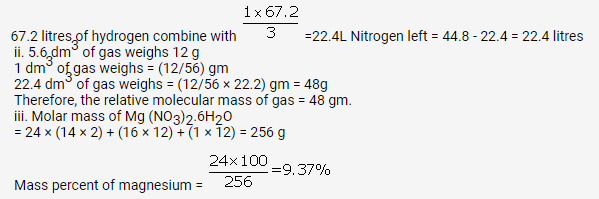
Solution 51.


Solution 52.

Solution 42.
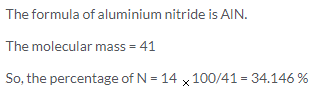
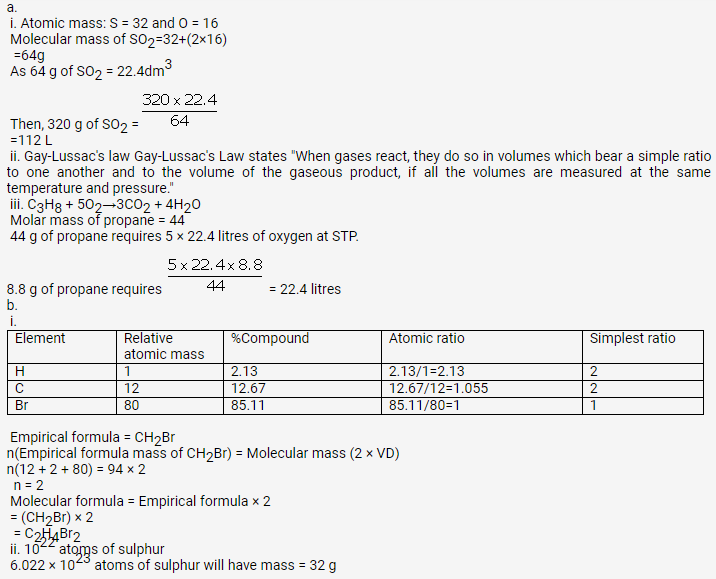
Solution 48.
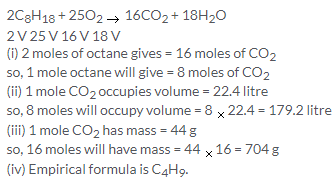
Solution 49.
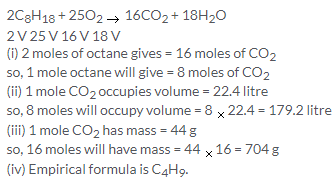
Solution 50.
(a) (i) element % atomic mass at. ratio simple ratio
C 14.4 12 1.2 1
H 1.2 1 1.2 1
Cl 84.5 35.5 2.38 2
Empirical formula = CHCl2
(ii) Empirical formula mass = 12+1+71= 84 g
Since molecular mass = 168 so, n = 2
so, molecular formula = (CHCl2)2 = C2H2Cl4
(b) (i) C + 2H2SO4 → CO2 + 2H2O + 2SO2
1 V 2 V 1 V 2 V
196 g of H2SO4 is required to oxidized = 12 g C
So, 49 g will be required to oxidise = 49 x 12/196 = 3 g
(ii) 196 g of H2SO4 occupies volume = 2 x 22.4 litres
So, 49 g H2SO4 will occupy = 2 x 22.4 x 49/196 = 11.2 litre
i.e. volume of SO2 = 11.2 litre
More Resources for Selina Concise Class 10 ICSE Solutions

![]()