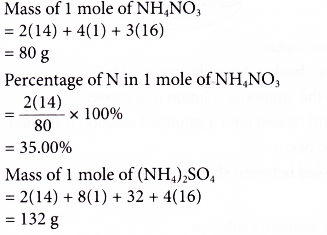Selina Concise Chemistry Class 10 ICSE Solutions Study of Compounds – Study of Compounds – Ammonia
APlusTopper.com provides step by step solutions for Selina Concise ICSE Solutions for Class 10 Chemistry Chapter 9 Study of Compounds Ammonia. You can download the Selina Concise Chemistry ICSE Solutions for Class 10 with Free PDF download option. Selina Publishers Concise Chemistry for Class 10 ICSE Solutions all questions are solved and explained by expert teachers as per ICSE board guidelines.
Download Formulae Handbook For ICSE Class 9 and 10
ICSE SolutionsSelina ICSE Solutions
Selina ICSE Solutions for Class 10 Chemistry Chapter 9 Study of Compounds – Ammonia
Exercise Intext 1
Solution 1.

Solution 2.
The different forms of ammonia:
Gaseous ammonia(dry ammonia gas)
Liquid ammonia
Liquor ammonia fortis
Laboratory bench reagent
Solution 3.
Formula of liquid ammonia is: NH3.
Liquid ammonia is liquefied ammonia and is basic in nature. It dissolves in water to give ammonium hydroxide which ionizes to give hydroxyl ions.

Solution 4.

Solution 5.
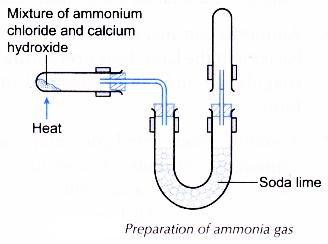
(a) Lab preparation of ammonia:
2NH4Cl + Ca(OH)2 → CaCl2 +2H2O +2NH3
(b) The ammonia gas is dried by passing through a drying tower containing lumps of quicklime (CaO).
(c) Ammonia is highly soluble in water and therefore it cannot be collected over water.
Solution 6.
The drying agent used is CaO in case of ammonia.
Other drying agents like P2O5 and CaCl2 are not used. As ammonia being basic reacts with them.
6NH3 + P2O5 + 3H2O → 2(NH4)3PO4
CaCl2 +4NH3 → CaCl2.4NH3
Solution 7.
The substance A is Ammonium chloride and ‘B’ is Ammonia.
Reaction:
2NH4Cl + Ca(OH)2 → CaCl2 + 2H2O + 2NH3
Solution 8.

Solution 9.
(a) Ammonium compounds being highly soluble in water do not occur as minerals.
(b) Ammonium nitrate is not used in the preparation of ammonia as it is explosive in nature and it decomposes forming nitrous oxide and water vapours.
(c) Conc. H2SO4 is not used to dry ammonia, as ammonia being basic reacts with them.
2NH3 + H2SO4 → (NH4)2SO4
Solution 10.
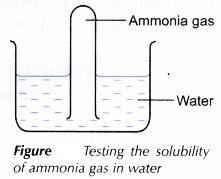
Preparation of Aqueous Ammonia: An aqueous solution of ammonia is prepared by dissolving ammonia in water. The rate of dissolution of ammonia in water is very high; therefore, back suction of water is possible. To avoid this, a funnel is attached to the outer end of the delivery tube with rubber tubing.
Procedure: Water is taken in a container and only a small portion of the mouth of funnel is dipped in water.
As ammonia dissolves in water at a higher rate than its production in the flask, the pressure in the funnel above water level decreases for a moment and water rushes into the funnel. As a result, the rim of the funnel loses its contact with water. Since, ammonia produced pushes the water down, the funnel comes in contact with water again. In this way, ammonia dissolves in water without back suction of water.

Exercise 1
Solution 1.
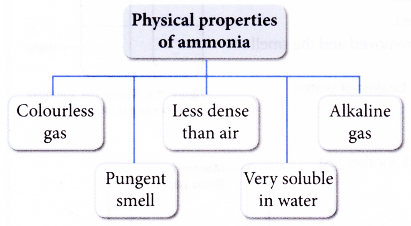
Physical properties of ammonia are:
Color: Colourless
Odour: Strong, Pungent chocking smell
Taste: Slightly bitter (alkaline ) taste
Physiological action: Non-Poisonous
Density: V.D = 8.5 Lighter than air
Nature: Alkaline
Liquefaction: easily liquefied at 10oC by compressing it at 6 atm. Pressure
Boiling Point: Liquid ammonia boils at -33.5oC
Freezing Point: Solid NH3 melts at -77.7oC
Solubility: Highly soluble in water, 1vol of water dissolves about 702 vols. of ammonia at 20oC and 1 atm. pressure.
Reaction:
2NH4Cl + Ca(OH)2 → CaCl2 + 2H2 + 2NH3

Solution 2.
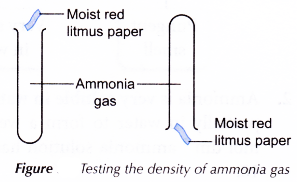
Ammonia is less dense than air. By Fountain Experiment we demonstrate the high solubility of ammonia gas in water.
Balanced equation for the reaction between ammonia and sulphuric acid is:
2NH3 + H2SO4 → (NH4)2SO4
Solution 3.
(a) Ammonia is basic in nature.
(b) Copper oxide because CuO is less reactive can be reduced by C, CO or by hydrogen whereas Al2O3, Na2O, MgO are reduced by electrolysis.
Solution 4.
(a) The formula of the compound is Mg3N2.
(b) Balanced equation :
Mg3N2 + 6 H2O → 3Mg(OH)2 + 2 NH3
(c) Ammonia is a reducing agent and reduces less active metal oxide to its respective metal.
Solution 5.
Reducing property.
Solution 6.
When a piece of moist red litmus paper is placed in a gas jar of ammonia it turns blue.
Solution 7.
(a) The gas is ammonia.
(b) The formula is NH3.
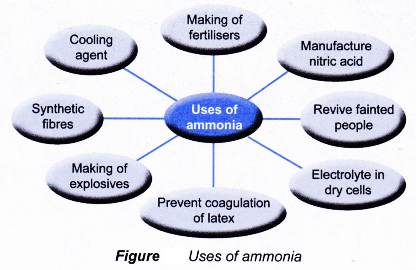
(c) Uses of ammonia:
- It is used in the industrial preparation of nitric acid by Ostwald process.
- It is used in the manufacture of fertilizers such as ammonium sulphate, ammonium nitrate, ammonium phosphate.
- It is used in the manufacture sodium carbonate by Solvay process.
NaCl + NH3 + CO2 + H2O → NaHCO3 + NH4Cl
Solution 8.
Equation:
CuSO4 + 2NH4OH → Cu(OH)2 ↓ + NH4]2SO4
pale blue
Ammonia solution in water gives a blue precipitate when it combines with a solution of copper salt.
The pale blue precipitate of copper hydroxide dissolves in excess of ammonium hydroxide forming tetraamine copper[II] sulphate, an azure blue(deep blue)soluble complex salt.
Cu(OH)2 +(NH4)2SO4 +2NH4OH → [Cu(NH3)4]SO4 + 4H2O
Solution 9.

Solution 10.
(a) Liquid ammonia takes a lot of energy to vaporize .This heat is taken from the surrounding bodies which are consequently cooled down. Thus it is used as a refrigerant in ice plant.
(b) Ammonia emulsifies or dissolves fats, grease so it is used to remove grease from woolen clothes.
(c) Aqueous solution of ammonia gives pungent smell because of the presence of ammonia.
(d) Aqueous ammonia when dissolved in water breaks into ions which help in the conductance of electricity.
![]()
Solution 11.
(a) AlN + 3H2O → Al(OH)3 +NH3
(b) 2NH3 + 3PbO → 3Pb + 3H2O + N2
(c) 8NH3 +3Cl2 → N2 + 6NH4Cl
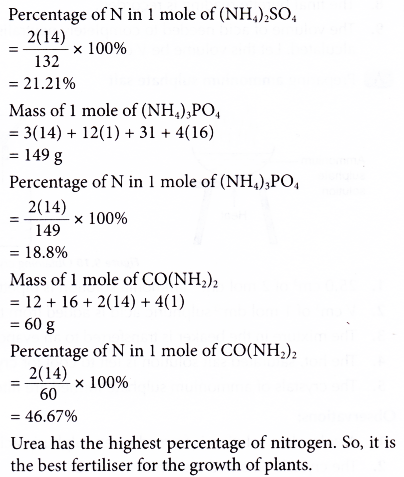
(d) 2NH3 + CO2 → NH2CONH2 + H2O
(i) Ammonia act as reducing agent is explained by equation (c).
(ii) Urea the nitrogenous fertilizer is prepared from equation (d).
Solution 12.
(a) A Dirty green precipitate of Fe(OH)2 is obtained when ammonium hydroxide is added to ferrous sulphate.
(b) Liquid ammonia is liquefied ammonia.
(c) Finely divided Iron is used in Haber process.
(d) Quicklime is a drying agent for NH3.
(e) Ammonium salts when heated with caustic alkali.
Solution 13.
(a) Dirty green ppt. of Ferrous hydroxide is formed which is insoluble in excess of NH4OH.
FeSO4 + 2NH4OH → [NH4]2SO4 + Fe(OH)2
(b) Reddish brown ppt. of ferric hydroxide is formed which is insoluble in ammonium hydroxide.
FeCl3 + 3NH4OH → 3NH4Cl + Fe(OH)3
(c) White ppt. of lead hydroxide is formed which is insoluble in NH4OH.
Pb(NO3)2 + 2NH4OH → 2NH4NO3 + Pb(OH)2
(d) White gelatinous ppt. of Zinc hydroxide is formed which is soluble in NH4OH.
Zn(NO3)2 + 2NH4OH → 2NH4NO3 + Zn(OH)2
Solution 14.
When correct amount of ammonium hydroxide is added drop wise to solutions of the metallic salts, ppts. (coloured generally) are formed. They help us to identify their metal ions.
Two equations:
FeSO4 +2NH4OH → (NH4)2SO4 + Fe (OH)2
(Green) (Dirty green)
shows the presence of Fe+2 ion.
FeCl3 + 3NH4OH → 3NH4Cl + Fe (OH)3
(Brown) (Reddish brown)
shows the presence of Fe+3 ion.
Solution 15.
![]()
NH4Cl on strong heating sublimes to form dense white fumes which condense to white powdery mass on cooler parts of the tube whereas no white fumes on heating NaCl.
(b) When ammonium hydroxide is added drop wise to solution to be tested.
Ferrous salt gives dirty green ppt.
Ferric salt gives reddish brown ppt of their hydroxides.
(c) (NH4)2SO4 on warming with NaOH sol. gives NH3 gas. Sodium sulphate does not liberate NH3 gas.
Solution 16.

Solution 17.
(a) In the presence of Platinum at 800oC, ammonia reacts with oxygen to give nitric oxide and water vapour.
Procedure:
Pass dry ammonia gas and oxygen gas through inlets over heated platinum placed in the combustion tube, which in the heated state emits reddish glow.

Observation:
Reddish brown vapours of nitrogen dioxide are seen in the flask due to the oxidation of nitric oxide.
The platinum continues to glow even after the heating is discontinued since the catalytic oxidation of ammonia is exothermic.
(b) Two reactions to show reducing property of ammonia are:
8NH3 +3Cl2 → N2 + 6NH4Cl
2NH3 +3CuO → 3Cu + 3H2O +N2
Solution 18.
(i) Neutralization
NH3 + HCl → NH4Cl
(ii) Thermal dissociation
NH4Cl → NH3 + HCl
(iii) Ammonia
NH4Cl + NaOH → NH3 + NaCl + H2O
Solution 19.
(a) Ammonia
(b) Hydrogen chloride and chlorine gas.
(c) (i) Ammonium chloride
(i) Ammonium nitrate
(ii) Ammonium carbonate
(d) Acidic gas: HCl
Basic gas: Ammonia
Neutral gas: NH4Cl
(e) Silver chloride
(f) Nitrogen
(g) Magnesium nitride
(h) Lead oxide
(i) Ammonium chloride
Solution 20.
CuSO4 + 2NH4OH → (NH4)2SO4 + Cu(OH)2 [Pale blue]
The cation present in solution B is Copper (Cu+2).
The colour of solution B is Blue.
The pale blue precipitate of copper hydroxide dissolves in excess of ammonium hydroxide forming tetraamine copper[II] sulphate, an azure blue(deep blue) soluble complex salt.
Cu(OH)2 + (NH4)2SO4 +2NH4OH → [Cu(NH3)4]SO4 + 4H2O
Solution 21.
Three ways in which ammonia gas can be identified is:
- It has a sharp characteristic odour.
- When a glass rod dipped in HCl is brought in contact with the gas white colour fumes of ammonium chloride are formed.
- It turns moist red litmus blue, moist turmeric paper brown and phenolphthalein solution pink.
Solution 22.
(a) Mg3N2 + 6H2O 3Mg(OH)2 + 2NH3
(b) 2NH3 + 3CuO 3Cu + 3H2O + N2
Ammonia acts as a reducing agent. It reduces metallic oxide to give metals, water vapour and nitrogen.
(c) 8NH3 +3Cl2 N2 + 6NH4Cl
(d) 4 NH3 +5O2 6H2O + 4NO +Heat
Ostwald process starts with the catalytic oxidation of ammonia to manufacture nitric acid in the presence of catalyst platinum.
Solution 23.
As the ‘A’ turns red litmus blue it is a base. Now the gas ‘A’ combines with ‘B’ in presence of Catalyst to give colourless gas Nitrogen monoxide. It reacts with oxygen to give brown gas which is Nitrogen dioxide.
A= NH3
B= O2
C=NO
D=NO2

Solution 24.

Solution 25.
(a) The main refrigerants used are Freon chlorofluorocarbons (CFC). They deplete ozone layer. The chlorofluorocarbons are decomposed by ultraviolet rays to highly reactive chlorine which is produced in the atomic form.
This causes depletion of ozone layer and chlorine monoxide so formed reacts with atomic oxygen and produces more chlorine free radicals.
ClO + O → Cl + O2
Again this free radical destroys ozone and the process continues thereby giving rise to ozone depletion.
(b) Liquid ammonia can be used as a refrigerant, as an alternative for chlorofluorocarbons.
(c) Advantages of ammonia as refrigerant:
- Ammonia is environmentally compatible. It does not deplete ozone layer and does not contribute towards global warming.
- It has superior thermodynamic qualities as a result ammonia refrigeration systems use less electricity.
Ammonia has a recognizable odour and so leaks are not likely to escape.
Solution 26.
Disadvantages of ammonia as a refrigerant are as follows:
- It is not compatible with copper, so it cannot be used in any system with copper pipes.
- It is poisonous in high concentration although it is easily detectable due to its peculiar smell and since it is less dense than air it goes up in the atmosphere not affecting the life too much on earth.
Solution 27.
(a) Explosive: ammonium nitrate
(b) Medicine: ammonium carbonate
(c) Fertilizers: ammonium sulphate
(d) Laboratory reagent: ammonia solution
Solution 28.
(a) Dry air free from carbon dioxide and dry ammonia from Habers process.
(b) The catalyst used in the process is Platinum.
(c) The oxidizing agent used in the process is oxygen.
(d) Ratio of ammonia and air is 1:10.
(e) Quartz is acid resistant and when packed in layers help in dissolving nitrogen dioxide uniformly in water.
Solution 29.

Solution 30.

Solution 1 (2003).
(a) Mg3N2 +6H2O → 3Mg(OH)2 + 2NH3
(b) Ammonia gas is collected in inverted gas jars by the downward displacement of air.
(c) Ammonia is not collected over water because it is highly soluble in water.
(d) Quicklime is used as a drying agent for ammonia.
Solution 1 (2004).

Solution 1 (2005).
(a) It is the basic nature of ammonia molecule.
(b) Hydroxyl ion (NH3 + H2O → NH4+ + OH–)
(c) The red litmus paper turns blue in the solution.
Solution 1 (2006).
Pb(NO3)2+ NH4OH → 2NH4NO3+Pb(OH)2
The chalky white ppt. of lead hydroxide is formed.
Solution 1 (2007).
(a) HCl gas is more dense [V.D.=18.25,V.D. of ammonia =8.5] and it is collected by the upward displacement of air.
(b) NH3 + HCl → NH4Cl
Solution 2 (2005).

Solution 2 (2007).
Balanced equation:
(a) 2NH3 + 3CuO → 3Cu + 3H2O + N2
(b) 2NH3 + 3Cl2 → N2 + 6HCl
Solution 2 (2008).
Magnesium Nitride
More Resources for Selina Concise Class 10 ICSE Solutions