How is Sulfuric Acid Made?
Manufacture of sulphuric acid in industry:
- In industry, sulphuric acid is manufactured by Contact process.
- Sulphur, air or oxygen and water are the raw materials for the manufacture of sulphuric acid.
- The Contact process consists of three stages:
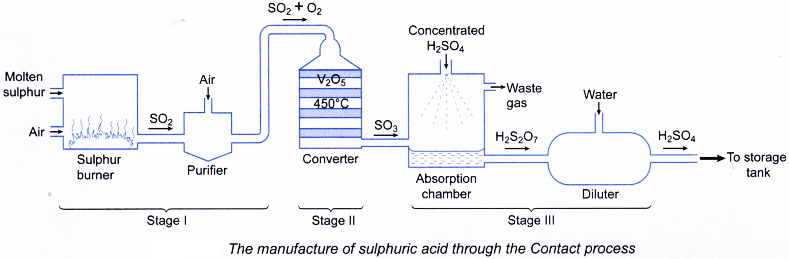
Stage I: Production of sulphur dioxide
(Sulphur → Sulphur dioxide)
Stage II: Conversion of sulphur dioxide to sulphur trioxide
(Sulphur dioxide → Sulphur trioxide)
Stage III: Production of sulphuric acid
(Sulphur trioxide → Sulphuric acid) - Stage I: Production of sulphur dioxide in the furnace
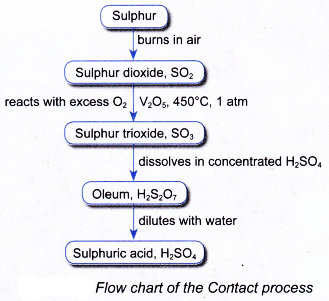
(a) Sulphur is burnt in air to produce sulphur dioxide.
S(s) + O2(g) → SO2(g)
(b) Sulphur dioxide can also be produced by burning metal sulphides such as zinc sulphide and lead sulphide.
2ZnS(s) + 3O2(g) → 2SO2(g) + 2ZnO(s)
2PbS(s) + 3O2(g) → 2SO2(g) + 2PbO(s)
(c) The sulphur dioxide is then mixed with excess air and dried. The mixture is next purified in order to remove impurities such as arsenic compounds. - Stage II: Conversion of sulphur dioxide to sulphur trioxide in the converter
(a) Pure and dried mixture of sulphur dioxide and oxygen is passed through a converter.
(b) The optimum conditions used in the converter are as follows:
(i) Temperature: 450°C
(ii) Pressure: 1 atmosphere
(iii) Catalyst: Vanadium(V) oxide, V2O5
(c) A high yeild of 95% of sulphur trioxide is produced.
2SO2(g)+ O2(g) → 2SO3(g) - Stage III: Production of sulphuric acid in absorber and diluter
(a) The sulphur trioxide is dissolved in concentrated sulphuric acid to produce oleum, H2S2O7 in the absorber.
SO3(g) + H2SO4(aq) → H2S2O7(l)
(b) The oleum is then diluted with water to produce concentrated sulphuric acid of about 98% in the diluter.
H2S2O7(l) + H2O(l) → 2H2SO4(aq) - The diagrammatic steps for the production of sulphuric acid is shown in Figure.
- The sulphur trioxide produced can react with water to form sulphuric acid.
SO3 + H2O → H2SO4
This is not done in industry because the reaction
(a) produces a lot of heat and may explode
(b) produces acidic fume which is difficult to condense and may pollute the air - The manufacture of sulphuric acid in industry is shown in the flow chart in Figure.
People also ask
- How Acid rain is formed equations?
- What is the Haber process used for?
- What are the physical properties of ammonia?
- Uses of ammonia in our daily life
Uses of sulphuric acid in daily life
Sulphuric acid is used to manufacture almost all products. Some of the examples are as follows.
- Fertilisers
- Paint pigments
- Detergents
- Synthetic fibres
- Electrolyte in car batteries
- Cleaning metals
- Plastics
- Other chemicals
- Almost one third of sulphuric acid is used to manufacture chemical fertilisers.
- Ammonium sulphate fertiliser is prepared from the reaction between sulphuric acid with aqueous ammonia.
H2SO4(aq) + 2NH3(aq) → (NH4)2SO4(aq) - Potassium sulphate fertiliser is prepared from the reaction between sulphuric acid with potassium hydroxide solution.
H2SO4(aq) + 2KOH(aq) → K2SO4(aq) + 2H2O(l) - Calcium hydrogen phosphate, Ca(H2P04)2 (superphosphate) is formed when sulphuric acid reacts with calcium phosphate.
2H2SO4(aq) + Ca3(PO4)2(s) → Ca(H2PO4)2(aq) + 2CaSO4(s)
- Ammonium sulphate fertiliser is prepared from the reaction between sulphuric acid with aqueous ammonia.
- Neutralisation of sulphuric acid with barium hydroxide solution produces barium sulphate which is used as white pigment in paint. Sulphuric acid is used to make titanium(IV) oxide. This white powder is used in the manufacture of pigment.
- Concentrated sulphuric acid reacts with by-products of petroleum to form sulphonic acid by a process called suphonation. Neutralisation of sulphonic acid with an alkali produces detergents.
- Synthetic fibres like rayon is produced by the reaction of sulphuric acid with cellulose threads soaked in alkaline solution.
- Dilute sulphuric acid is used as electrolyte in lead-acid accumulator in car batteries.
- Before electroplating, sulphuric acid is used for cleaning metals to remove the surface oxides.
- Sulphuric acid is used to manufacture many types of plastics.
- Sulphuric acid is also used to manufacture other chemicals like insecticides, pharmaceuticals, tartaric acid and explosives.
Sulphuric acid is also used in laboratory in schools as follows:
(a) As drying agent
(b) As dehydrating agent
(c) As catalyst
(d) As strong acid