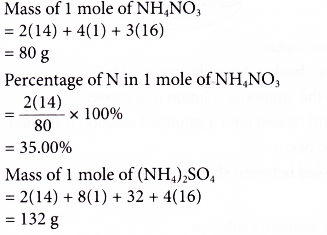Uses of ammonia in our daily life
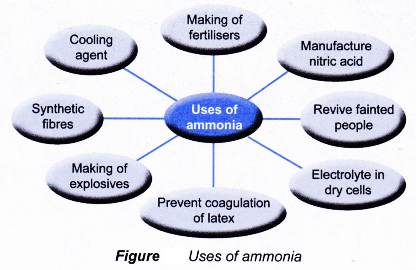
Uses of ammonia:
- Ammonia is produced industrially as an intermediate compound and as raw material for many other chemical processes.
- The main uses of ammonia are as follows:
(a) To manufacture nitrogenous fertilisers needed for plant growth
(b) As raw material for the manufacture of nitric acid
(c) As cooling agent in refrigerators
(d) To produce ammonium chloride used as electrolyte in dry cells
(e) To prevent coagulation of latex
(f) To make synthetic fibres such as nylon
(g) As smelling salts to revive people who have fainted
(h) Making of explosives
- The nitrogenous fertilisers are actually ammonium salts obtained from neutralisation of ammonia with different acids. These fertilisers include:
(a) Ammonium phosphate
The reaction of ammonia with phosphoric acid produces ammonium phosphate.
2NH3(aq) + H3PO4(aq) → (NH4)2HPO4(aq)
Ammonium phosphate is a good fertiliser as it provides two important nutrients, nitrogen and phosphorus.
(b) Ammonium nitrate
Ammonia reacts with nitric acid to produce ammonium nitrate.
NH3(aq) + HNO3(aq) → NH4NO3(aq)
(c) Ammonium sulphate
Ammonia reacts with sulphuric acid to produce ammonium sulphate.
2NH3(aq) + H2SO4(aq) → (NH4)2SO4(aq)
(d) Urea
Ammonia reacts with carbon dioxide at 200°C and atmospheric pressure of 200 to produce urea.
2NH3(g) + CO2(g) → CO(NH2)2(s) + H2O(l)
Urea has the highest percentage of nitrogen and is very suitable for plant growth. - Ammonia is used to make nitric-acid through Ostwald process. This process involves three stages.
(a) Ammonia is oxidised to nitrogen monoxide in the presence of platinum as the catalyst.
4NH3(g) + 5O2(g) → 4NO(g) + 6H2O(l)
(b) Nitrogen monoxide is further oxidised to nitrogen dioxide.
2NO(g) + O2(g) → 2NO2(g)
(c) Nitrogen dioxide and oxygen dissolve in water to form nitric acid.
4NO2(g) + O2(g) + 2H2O(1) → 4HNO3(aq) - Liquid ammonia is used as cooling agent in refrigerators because ammonia is highly compressible and has a high heat capacity.
- Ammonia can neutralise the acid produced by bacteria in the latex, thus preventing latex from coagulating and can be exported in the liquid form.
- Ammonia reacts with hydrochloric acid to form ammonium chloride. Ammonium chloride is used as electrolyte in dry cells.
NH3(aq) + HCl(aq) → NH4Cl(aq) - Ammonia reacts with nitric acid to form ammonium nitrate which is used as explosive. Nitric acid can be used to make explosives such as TNT.
People also ask
- What is the Haber process used for?
- What are the physical properties of ammonia?
- How is Sulfuric Acid Made?
- Uses of sulphuric acid in daily life
- How Acid rain is formed equations?
Ammonium fertilisers
- Nitrogen is used by plant to make protein. Protein is important for the growth of plant. Other nutrients needed by plants include phosphorus, potassium, calcium and magnesium.
- Nitrogenous compounds are removed from the soil by plants; some are replaced naturally by bacteria. To restore the balance, nitrogenous fertilisers are added to the soil.
- Nitrogenous fertilisers include ammonium fertilisers which contain ammonium ions.
- In the soil, the ammonium ions are converted to nitrate ions by bacteria. This is because nitrogen can only be absorbed by plants in the form of soluble nitrate ions.
- Examples of ammonium fertilisers are:
(a) Ammonium nitrate, NH4NO3
(b) Ammonium sulphate, (NH4)2SO4
(c) Ammonium phosphate, (NH4)3PO4
(d) Urea, CO(NH2)2 - The effectiveness of ammonium fertilisers is determined by the percentage of nitrogen by mass in them.
- The fertiliser with a higher percentage of nitrogen is more effective for growth than those fertilisers with a low percentage of nitrogen.
- The percentage of nitrogen by mass can be calculated from the formulae of the fertilisers using the following formula.
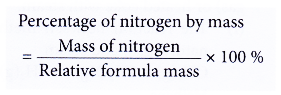
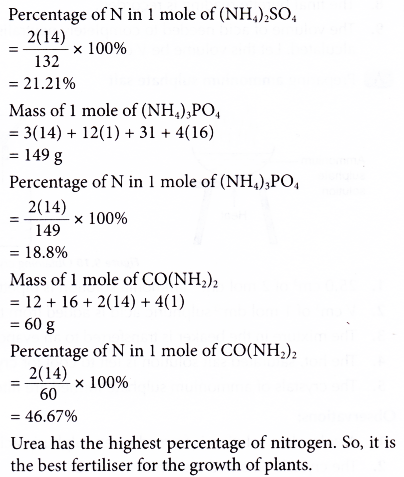
Example: The following are four fertilisers.
Ammonium nitrate, NH4N03
Ammonium sulphate, (NH4)2S04
Ammonium phosphate, (NH4)3P04 . Urea, CO(NH2)2
Which fertiliser is most suitable for the growth of plants? [Relative atomic mass: H, 1; C, 12; N, 14; O, 16; P, 31; S, 32]
Solution: