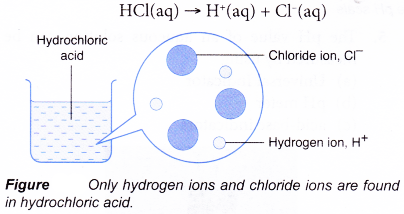
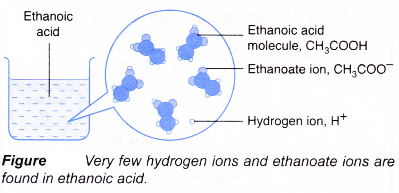
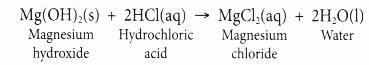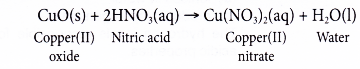What is the definition of a base in chemistry?
Bases
Bases are substances that are soapy to touch and bitter in taste.
Substances containing a base are called basic substances. Sodium hydroxide (NaOH) and calcium hydroxide [Ca(OH)2] are examples of bases used in the laboratory. Corn starch, fresh egg white, etc., are other examples of bases.
Bases may have a strong irritating odour and should be used with caution as they can harm the skin and eyes.

A base is a substance, usually the oxide or the hydroxide of a metal, which can react with an acid to produce salt and water.
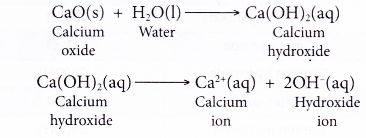
For example, sodium oxide (Na2O), calcium oxide (CaO), cupric oxide (CuO), iron oxides (FeO, Fe2O3 etc.), sodium hydroxide (NaOH) and calcium hydroxide (Ca(OH)2 are all bases.
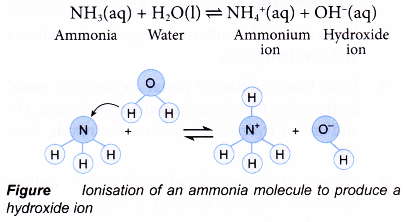
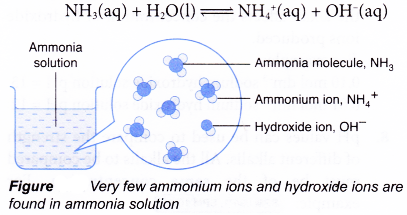
Certain substances are also called bases, though they do not fit into the above definition. For example, ammonia (NH3). It forms salt with an acid without giving water. So, it should not be treated as a base. But ammonium hydroxide (NH4OH), the aqueous solution of NH3, is a base as it reacts with an acid to give salt and water
NH4OH + HCl → NH4Cl + H2O
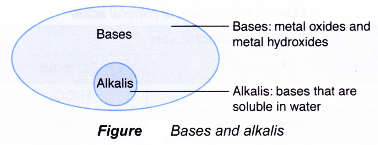
Alkalis :
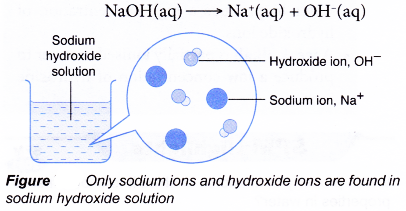
Bases that are soluble in water are called alkalis. For example, sodium hydroxide, potassium hydroxide, calcium hydroxide are soluble in water. Therefore, they are alkalis. But bases like copper hydroxide (Cu(OH)2 ferric hydroxide (Fe(OH)3), aluminium hydroxide (Al(OH)3 do not dissolve in water. They are, therefore, not alkalis.
Hence, all alkalis are bases, but all bases are not alkalis. Some of the bases are listed here in Table.
Oxides | Soluble hydroxides | Insoluble hydroxides |
Sodium monoxide (Na2O) | Sodium hydroxide (NaOH) | Ferric hydroxide (Fe(OH)3). |
Calcium oxide (CaO) | Potassium hydroxide (KOH) | Aluminium hydroxide (Al(OH)3) |
| Cupric oxide (CuO) ZnO | Calcium hydroxide (Ca(OH)2) Ammonium hydroxide NH4OH |
Lime water, baking soda and washing soda are all bases.
People also ask
- What is the definition of an acid and a base?
- What is the definition of an acid in chemistry?
- Classification of Acids
- Preparation of Acids
- What are the chemical properties of an acid?
- General Properties of Acids
- Uses of Acids
- Preparation of Bases
- General Properties of Bases
- What determines a Strong Base and a Weak Base
- What are the uses of Bases
- How can we measure the strength of acids and alkalis?
- How to calculate concentration of acids and alkalis?
- How do you prepare a standard solution?
- What is meant by a neutralization reaction?
- How does titration determine concentration?
- Relationship between pH values and molarity of acids and alkalis
- Concept of the pH Scale
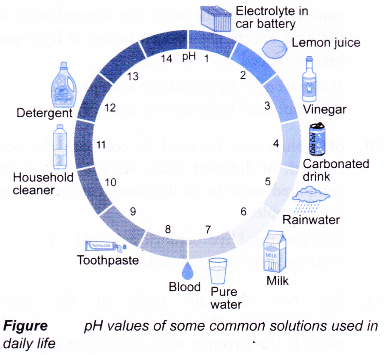
- Role of pH in everyday life
- What is the pH of a salt solution