Preparation of Acids
There are several methods for preparing acids. Some of them are discussed here.
1. Synthetic method : In the synthetic method, acids are prepared by direct combination of elements. For example, hydrogen and chlorine react together under the action of an electric spark to produce hydrogen chloride gas which is absorbed in water to give hydrochloric acid.
![]()
Similarly, sulphuric acid may be obtained from its elements as follows.
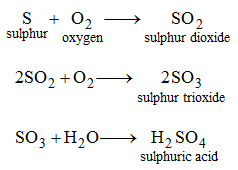
2. By dissolving acidic oxides in water : Some oxides dissolve in water to give acids. These oxides are called acidic oxides. For example, sulphur trioxide (SO3) dissolves in water to give H2SO4.
SO3 + H2O → H2SO4
Silimlarly, carbon dioxide (CO2) dissolves in water to produce carbonic acid (H2CO3).
CO2 + H2O → H2CO3
3. By the action of an acid on the salt of another acid : An acid having higher boiling point can react with the salt of an acid of lower boiling point to produce an acid. For example, NaCl is a salt of HCl. The boiling point of HCI is lower than that of H2SO4, When NaCl (salt of HCl) reacts with H2SO4, HCl is formed.
H2SO4 + NaCl → NaHSO4 + HCl
People also ask
- What is the definition of an acid and a base?
- What is the definition of an acid in chemistry?
- What is the definition of a base in chemistry?
- Classification of Acids
- What are the chemical properties of an acid?
- General Properties of Acids
- Uses of Acids
- Preparation of Bases
- General Properties of Bases
- What determines a Strong Base and a Weak Base
- What are the uses of Bases
- How can we measure the strength of acids and alkalis?
- How to calculate concentration of acids and alkalis?
- How do you prepare a standard solution?
- What is meant by a neutralization reaction?
- How does titration determine concentration?
- Relationship between pH values and molarity of acids and alkalis
- Concept of the pH Scale
- Role of pH in everyday life
- What is the pH of a salt solution