Classification of Acids
Depending upon the elements present, acids may be classified as follows.
(i) Oxyacid : Acids that contain both hydrogen and oxygen are called oxyacids. For example, nitric acid (HNO3), sulphuric acid (H2SO4) and phosphoric acid (H3PO4) are oxyacids.
(ii) Hydracid : Acids that contain hydrogen and other nonmetallic element(s), except oxygen, are called hydracids. For example, hydrochloric acid (HCl) and hydrocyanic acid (HCN) are hydracids.
Acids may also be classified as follows.
1. Organic and inorganic acids : All sour things that we use in our daily food contain acids. These acids are organic acids. Some of the common acids that are generally used in the laboratory are hydrochloric acid (HCl), sulphuric acid (H2SO4) and nitric acid (HNO3). These are inorganic acids, also called mineral acids. Hydrochloric acid is also present in the gastric juice in our stomach.
2. Concentrated and dilute acids : An acid solution may be concentrated or dilute depending upon the amount of the acid present in the solution. Concentrated and dilute solutions of acids are generally used in laboratories. Let us see what these acids are.
An acid is generally used as solution in water. When the solution contains a larger amount of the acid, it is said to be concentrated, whereas a dilute solution contains smaller amount of the acid.
Thus, concentrated and dilute solutions of an acid differ from each other only in the proportions of the acid and water in them.
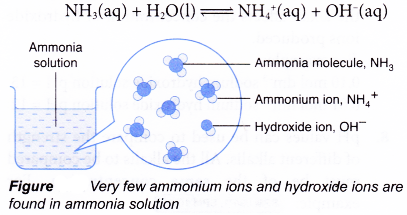
3. Strong and weak acids : The strength of an acid is determined by the amount of hydrogen ions (H+) that the acid provides when dissolved in water.
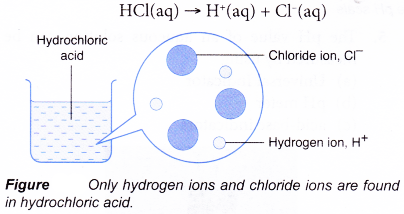
Some of the acids, when dissolved in water, get almost completely dissociated to provide hydrogen ions. These acids are called strong acids. For example, hydrochloric acid (HCl), nitric acid (HNO3) and sulphuric acid (H2SO4) are strong acids.
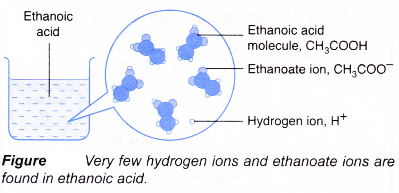
On the other hand, there are some acids which when dissolved in water, are only incompletely dissociated to give hydrogen ions. These are called weak acids. For example, carbonic acid (H2CO3) and acetic acid (CH3COOH) are weak acids.
People also ask
- What is the definition of an acid and a base?
- What is the definition of an acid in chemistry?
- What is the definition of a base in chemistry?
- Preparation of Acids
- What are the chemical properties of an acid?
- General Properties of Acids
- Uses of Acids
- Preparation of Bases
- General Properties of Bases
- What determines a Strong Base and a Weak Base
- What are the uses of Bases
- How can we measure the strength of acids and alkalis?
- How to calculate concentration of acids and alkalis?
- How do you prepare a standard solution?
- What is meant by a neutralization reaction?
- How does titration determine concentration?
- Relationship between pH values and molarity of acids and alkalis
- Concept of the pH Scale
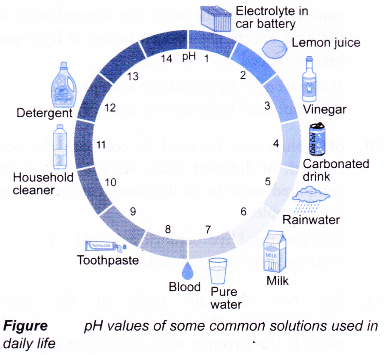
- Role of pH in everyday life
- What is the pH of a salt solution