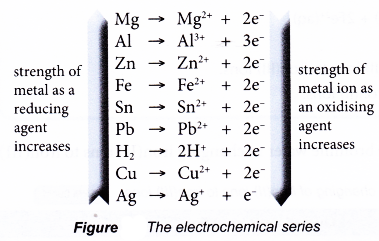
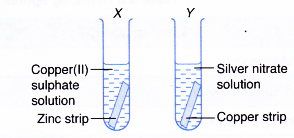
Displacement of Halogen from Halide Solution
- Generally, halogens are good electron acceptors and therefore are good oxidising agents.
- (a) When going down Group 17, the size of the halogen atoms increases. The nucleus is further away from the outermost occupied shell.
(b) Hence, the electronegativity of halogens or their ability to accept electrons to form negatively-charged halide ions decreases down the group.
(c) As a result, the strength of halogens as oxidising agents decreases down the group. - Chlorine, bromine and iodine are three
commonly used halogens in the laboratory. Each of them gives different colour in aqueous solution. However, the colour changes slightly with concentration. - Therefore, the presence of the halogens is confirmed using an organic solvent such as 1,1,1-trichloroethane, CH3CCl3.
- This is done by mixing thoroughly 1,1,1-trichloroethane to an aqueous solution of a halogen. Two layers will be formed whereby the denser 1,1,1-trichloroethane layer will be at the bottom and the less dense aqueous layer will be at the top.
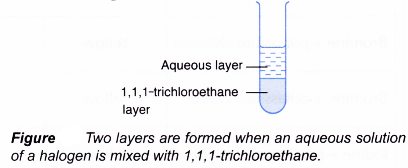
Based on the colour of the 1,1,1-trichloroethane layer, the halogen present is identified.
| Halogen | Colour of halogen in aqueous solution | Colour of halogen in 1,1,1-trichloroethane |
| Chlorine | Pale yellow or colourless | Pale yellow or colourless |
| Bromine | Brown, yellowish-brown or yellow, depending on concentration | Brown, orange or yellow, depending on concentration |
| Iodine | Brown, yellowish-brown or yellow, depending on concentration | Purple |
In a displacement of halogen, a more electronegative halogen displaces a less electronegative halogen from its halide solution.
- The halide ions of the less electronegative halogen act as the reducing agent. They lose their electrons and are oxidised to form halogen molecules.
- The electrons are accepted by the more electronegative halogen which acts as the oxidising agent. By doing so, the halogen undergoes reduction to form its halide ions.
- In short, there is an electron transfer from the halide ions of the less electronegative halogen to the more electronegative halogen.

People also ask
- What is a redox reaction?
- Changing of iron(II) ions to iron(III) ions and vice versa
- Redox reaction in the displacement of metals from its salt solution
- Redox Reactions by Transfer of Electrons at a Distance
- Rusting as a Redox Reaction
- The Reactivity Series of Metals Towards Oxygen
- Application of the reactivity series of metals in the extraction of metals
- Electrolytic and Chemical Cells
- Oxidation and Reduction in Electrolytic Cells
- Oxidation and Reduction in Chemical Cells
- How does a voltaic cell work?
Displacement of Halogen from Halide Solution Experiment
Aim: To investigate the oxidation and reduction in displacement of halogen.
Materials: Chlorine water, bromine water, iodine solution, 0.5 mol dm-3 potassium chloride solution, 0.5 mol dm-3 potassium bromide solution, 0.5 mol dm-3 potassium iodide solution, 1,1,1-trichloroethane.
Apparatus: Test tubes, test tube rack.
Procedure:
- 2 cm3 of 0.5 mol dm-3 potassium bromide solution is poured into a test tube.
- 2 cm3 of chlorine water is added to the test tube and the mixture is shaken thoroughly.
- 2 cm3 of 1,1,1 -trichloroethane is added to the mixture. The mixture is shaken thoroughly.
- After a few seconds, the colour of the aqueous and the 1,1,1-trichloroethane layers are observed.
- Steps 1 to 4 are repeated using the halogens and halide solutions as shown in Table 3.11.
Results:
| Mixture | Colour of aqueous layer | Colour of 1,1,1- trichloroethane layer | Inferences |
| Chlorine + potassium bromide | Yellow | Orange | Bromine is present. Displacement of bromine has occurred. |
| Chlorine + potassium iodide | Yellow | Purple | Iodine is present. Displacement of iodine has occurred. |
| Bromine + potassium chloride | Yellow | Orange | Bromine is present. No displacement reaction has occurred. |
| Bromine + potassium iodide | Yellow | Purple | Iodine is present. Displacement of iodine has occurred. |
| Iodine + potassium chloride | Yellow | Purple | Iodine is present. No displacement reaction has occurred. |
| Iodine + potassium bromide | Yellow | Purple | Iodine is present. No displacement reaction has occurred. |
Discussion:
- The results can be summarised as follows.

- Chlorine is more electronegative than bromine and iodine. Therefore,
(a) chlorine displaces bromine from potassium bromide solution. Chlorine acts as the oxidising agent, whereas bromide ions act as the reducing agent.
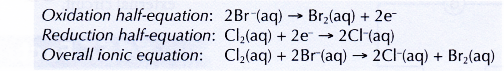
(b) chlorine displaces iodine from potassium iodide solution. Chlorine acts as the oxidising agent, whereas iodide ions act as the reducing agent.

- (a) Bromine is less electronegative than chlorine. Therefore, bromine cannot displace chlorine from potassium chloride solution.
(b) Bromine is more electronegative than iodine. Therefore, bromine displaces iodine from potassium iodide solution. Bromine acts as the oxidising agent, whereas iodide ions act as the reducing agent.

- Iodine is less electronegative than chlorine and bromine. Therefore,
(a) iodine cannot displace chlorine from potassium chloride solution.
(b) iodine cannot displace bromine from potassium bromide solution.
Conclusion:
A more electronegative halogen can displace a less electronegative halogen from its halide solution whereby the more electronegative halogen acts as the oxidising agent and the halide ions of the less electronegative halogen act as the reducing agent.