What are carbon compounds?
- Carbon compounds are compounds which contain the element carbon.
- These compounds contain carbon and hydrogen only or carbon and hydrogen in combination with a few other elements such as oxygen, sulphur, nitrogen, halogens and phosphorus.
Organic compounds
- Carbon compounds can be classified into two groups:
(a) Organic compounds
(b) Inorganic compounds - Chemists define organic compounds as carbon- containing compounds. However, some exceptions to this definition are
(a) oxides of carbon such as carbon monoxide, CO and carbon dioxide, CO2
(b) carbonates such as calcium carbonate, CaCO3
(c) hydrogen carbonates such as sodium hydrogen carbonate, NaHCO3
(d) cyanides such as potassium cyanide, KCN
(e) metallic carbides such as aluminium carbide, Al4C3 - Inorganic compounds include all non-carbon-containing compounds and the few carbon- containing compounds just mentioned.
- Table shows examples of organic and inorganic compounds found in nature.
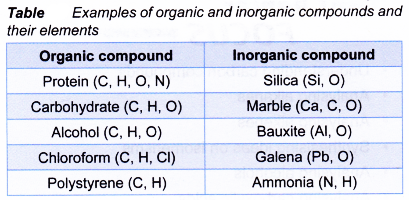
- Most organic compounds contain the elements carbon and hydrogen. Hence, complete combustion of organic compounds produces carbon dioxide and water.
- The following equation shows the complete combustion of glucose, C6H12O6.
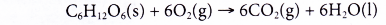
Complete combustion of organic compounds experiment
Aim: To investigate the complete combustion of organic compounds.
Materials: Ice, ethanol, palm oil, limewater, water.
Apparatus: Beaker, boiling tubes, rubber stoppers with two holes, delivery tubes, rubber tubing, filter funnel, filter pump, spirit lamp, Bunsen burner, thermometer, test tube holder, retort stand and clamps, wooden blocks.
Procedure:
- A spirit lamp is filled with some ethanol and the apparatus is set up as shown below.
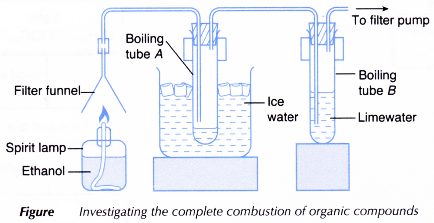
- The spirit lamp is lit to let the ethanol burn. At the same time, the filter pump is turned on.
- Changes taking place in boiling tubes A and B are noted and recorded.
- After about 15 minutes, the filter pump is turned off and boiling tube A is removed.
- The liquid inside boiling tube A is heated until it starts to boil. The boiling point of the liquid is recorded.
- Steps 1 to 5 are repeated using palm oil to replace ethanol.
Observations:
- The colourless liquid in boiling tube A boils at 100°C.
- The limewater turns milky.
Discussion:
- When an organic compound is burnt in excess oxygen,
(a) the carbon combines with oxygen to form carbon dioxide.
C + O2(g) → CO2(g)
(b) the hydrogen combines with oxygen to form water.
4H + O2(g) → 2H2O(g) - Carbon dioxide is detected through its ability to form a white precipitate with limewater.
- When a liquid boils at 100°C and 1 atmospheric pressure, it is pure water.
Conclusion:
Complete combustion of an organic compound produces carbon dioxide and water.
People also ask
- Chemical Properties of Carbon Compounds
- How are alkanes formed?
- What is an alkene in chemistry?
- What is an isomerism?
- What is alcohol and how is it made?
- How are carboxylic acids formed?
- How esters are formed?
- What are fats and oils?
- How palm oil is extracted?
- Order in Homologous Series
- What is the monomer of natural rubber?
- Which acid is used for coagulating rubber from latex?
- Classification of Hydrocarbons
- What is the homologous series of hydrocarbons?
- Properties and Uses of Ethanol
- Properties and Uses of Ethanoic Acid
Hydrocarbons
- Hydrocarbons are the simplest of all organic compounds, containing only carbon and hydrogen.
- Hydrocarbons are classified into two groups:
(a) Saturated hydrocarbon
(b) Unsaturated hydrocarbon - Saturated hydrocarbon molecules are made entirely of carbon-carbon single bonds. Saturated hydrocarbons contain only single covalent bonds.
- Unsaturated hydrocarbon molecules contain at least one carbon-carbon double or triple bond. Unsaturated hydrocarbons contain multiple bonds.
Example:
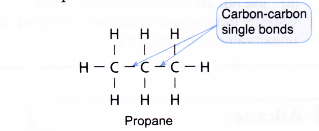
- The most common natural sources of hydrocarbons are petroleum, coal, natural gas and certain trees such as the rubber tree.
- Non-hydrocarbons are organic compounds in which some or all of the hydrogen atoms have been replaced by other atoms such as oxygen, nitrogen, phosphorus or halogens.
For example,

(a) sugar is a compound of carbon, hydrogen and oxygen.
(b) protein contains carbon, hydrogen, oxygen and nitrogen.
(c) tetrachloromethane contains carbon and chlorine.