How many Allotropes of Carbon are there
The property of an element to exist in two or more physical forms having more or less similar chemical properties but different physical properties is called allotropy. The different forms of the element are called allotropes. Allotropes form due to the difference in the arrangement of atoms in the molecules.
The allotropes of carbon are classified into two types. They are
• Amorphous forms
• Crystalline forms
Amorphous forms
Different amorphous allotropes of carbon are: Coal; Coke; Wood Charcoal; Animal charcoal; Lamp black; Gas carbon; Petroleum coke; Sugar charcoal.
Crystalline forms
Carbon atoms can arrange themselves into different hybridised chemical bonds. Therefore they exhibit different physical and chemical structures such as diamond and graphite. Carbon in solid phase can exist
in three crystalline allotropic forms: diamond, graphite and buckminsterfullerene.
Diamond and graphite form covalent network structures whereas buckminster fullerene has a molecular solid structure with discrete C60 molecules. As these crystalline allotropes differ in their structures, they
possess different physical properties.
Diamond
In diamond each carbon atom undergoes in its excited state sp3 hybridisation. Hence, each carbon atom has a tetrahedral environment. The three dimensional structure of diamond is as shown:
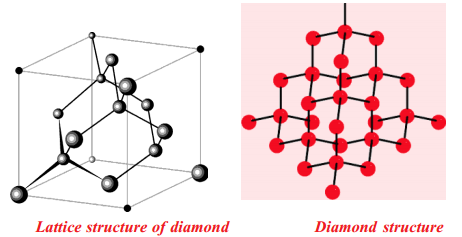 As C-C bonds are very strong any attempt to distort the diamond structure requires large amount of energy. Hence diamond is one of the hardest materials known.
As C-C bonds are very strong any attempt to distort the diamond structure requires large amount of energy. Hence diamond is one of the hardest materials known.
Graphite
Graphite forms a two dimensional layer structure with C-C bonds within the layers. There are relatively weak interactions between the layers.
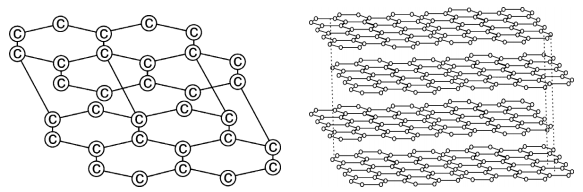 In the layer structure, the carbon atoms are in a trigonalplanar environment. This is consistent with each carbon atom in sp2 hybridisation.
In the layer structure, the carbon atoms are in a trigonalplanar environment. This is consistent with each carbon atom in sp2 hybridisation.
Interactions between the sp2 orbitals (overlaps) lead to the formation of C-C bonds. Each carbon atom is with one unhybridised ‘p’ orbital. The unhybridised ‘p’ orbitals interact to form a π system which is delocalised over the whole layer. The interactions known as London dispersion forces between the layers which are separated by a distance of 3.35 Ao are weakened by the presence of water molecules so that it is easy to cleave graphite.
For this reason graphite is used as lubricant and as the ‘lead’ in pencils.
When we write with a pencil, the interlayer attractions breakdown and leave graphite layers on the paper. Pencil marks are easy to remove from paper with an eraser because, the layers do not bind strongly to the paper.
Graphite is a good conductor of electricity because of the delocalised π electron system.
Buckminsterfullerene (60C)
Buckminsterfullerenes are molecules of varying sizes that are composed entirely of carbon. The arrangement of these molecules leads to the form of a hollow sphere, ellipsoid, or tube depending upon their orientations. Fullerenes are formed when vaporized carbon condenses in an atmosphere of an inert gas.
Buckyballs: Spherical fullerenes are also called buckyballs. Buckminsterfullerene (60C) contains nearly spherical 60C molecules with the shape of a soccer ball.
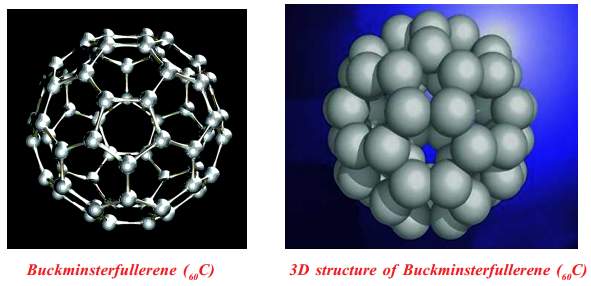 Fullerene, 60C molecule contains 12 pentagonal and 20 hexagonal faces on its soccer ball shape, and each carbon atom has sp2 hybridized orbitals.
Fullerene, 60C molecule contains 12 pentagonal and 20 hexagonal faces on its soccer ball shape, and each carbon atom has sp2 hybridized orbitals.
Fullerenes are under study for potential medicinal use – such as specific antibiotics to target resistant bacteria and even target certain cancer cells such as melanoma.
Read More:
- Binding of Carbon with other Elements
- Homologous Series of Hydrocarbons
- Catenation in Carbon
- Classification of Hydrocarbons
- sp3 Hybridized Carbon atom
- Hybridization of the Carbon atoms in Acetylene
- Versatile Nature of Carbon
- What are the Characteristics of Compounds
- Chemical Properties of Carbon Compounds
- Nomenclature of Carbon Compounds