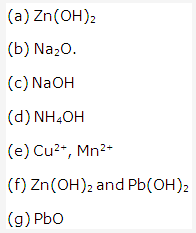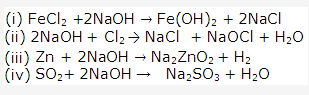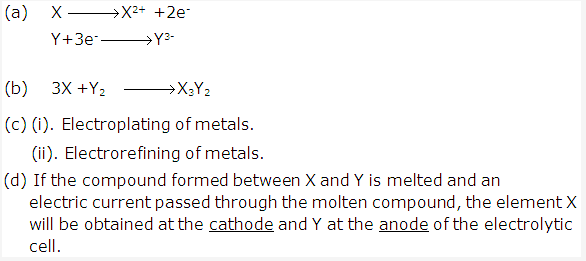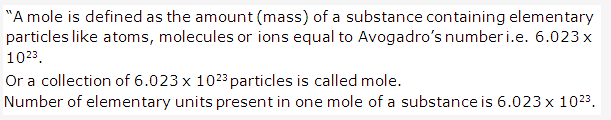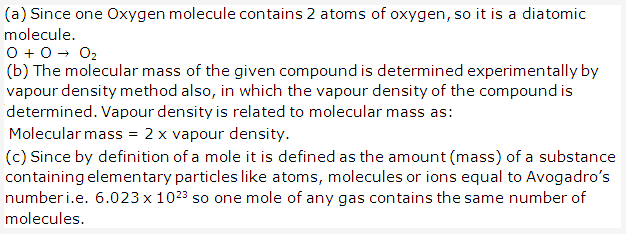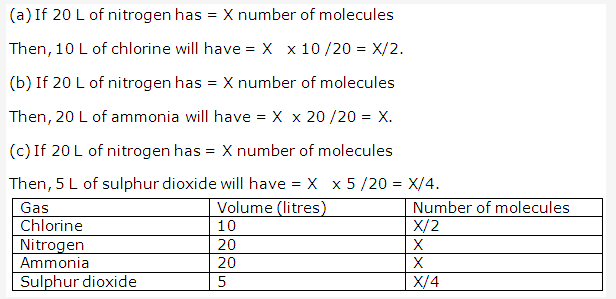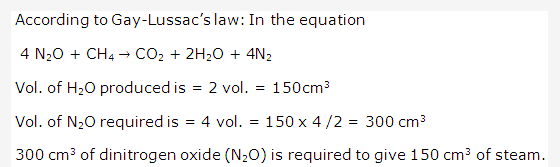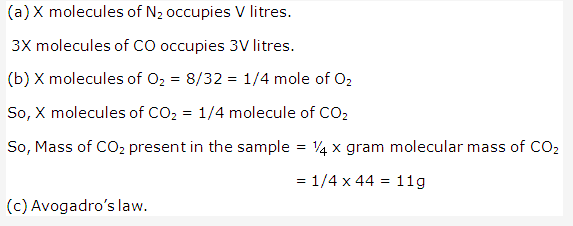Frank ICSE Solutions for Class 10 Chemistry – Study Of Acids, Bases and Salts
PAGE NO : 62
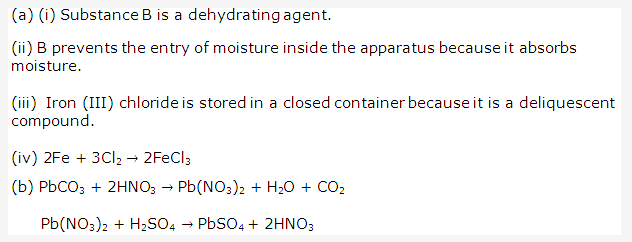
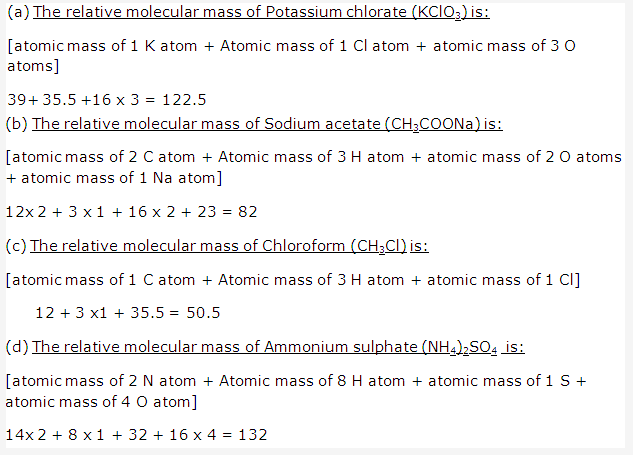
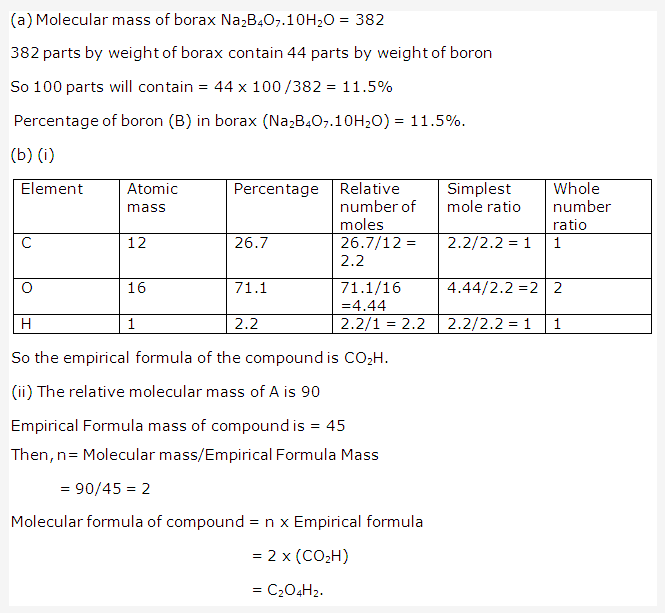
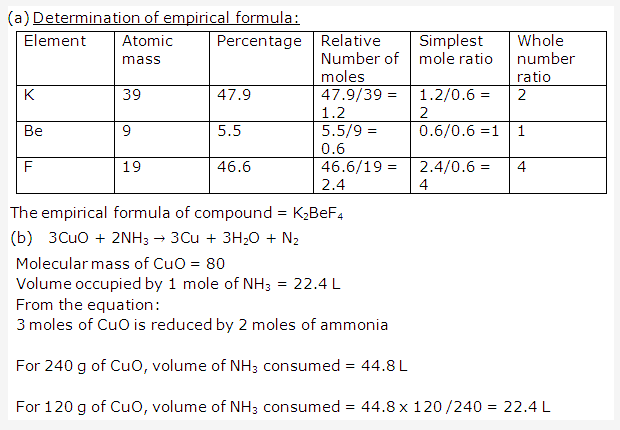
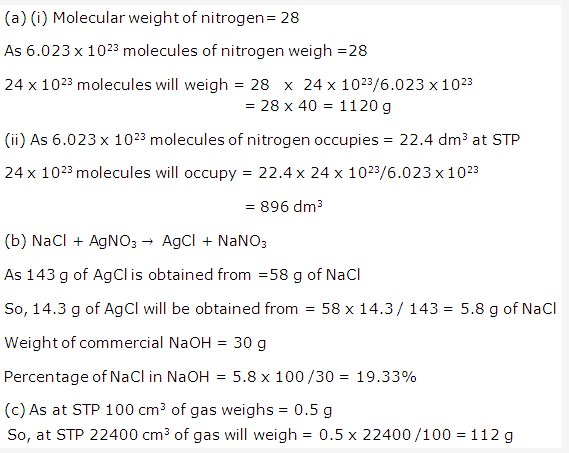
Solution 1:

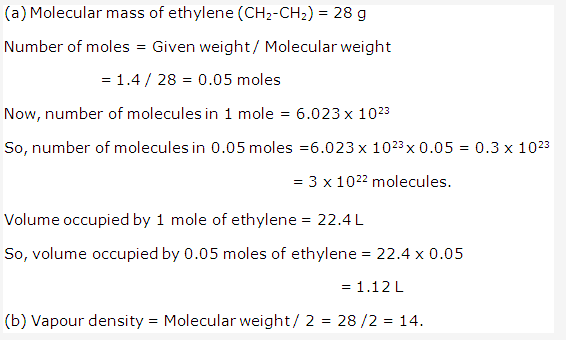
Solution 2:
- (i) Hydrogen chloride HCl
(ii) Nitric acid HNO3 - (i) Carbonic acid H2CO3
(ii) Oxalic acid (COOH)2 - (i) Sulphuric acid H2SO4
(ii) Hydrogen chloride HCl - (i) Carbonic acid H2CO3
(ii) Acetic acid
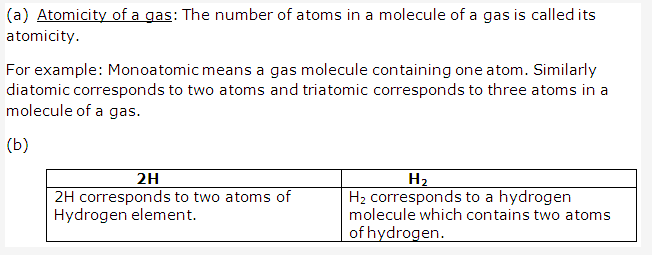
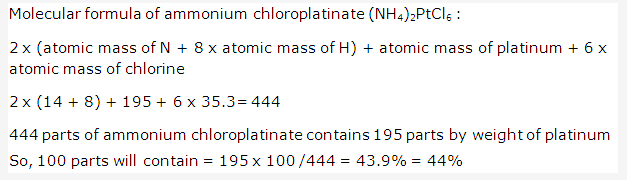
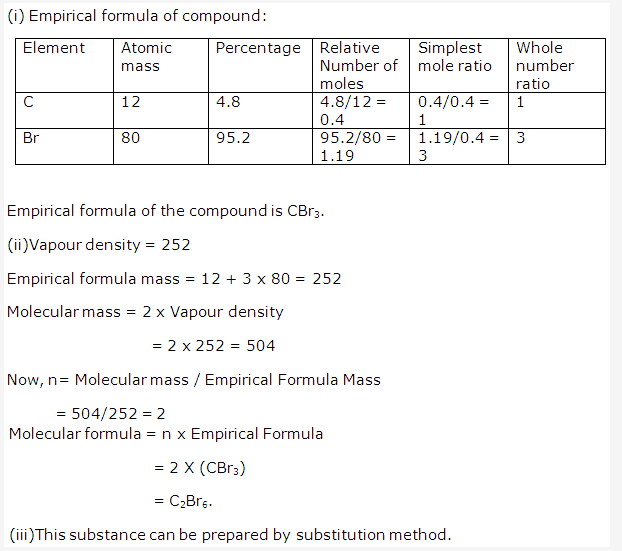
Solution 3:
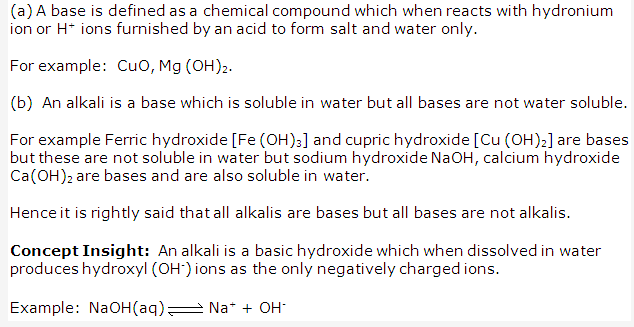
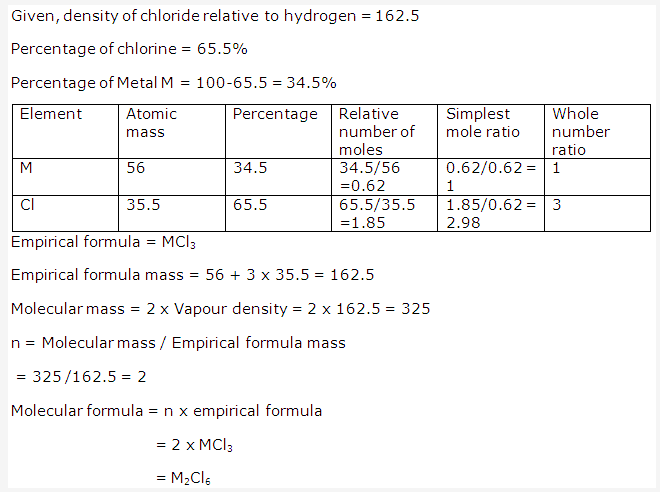
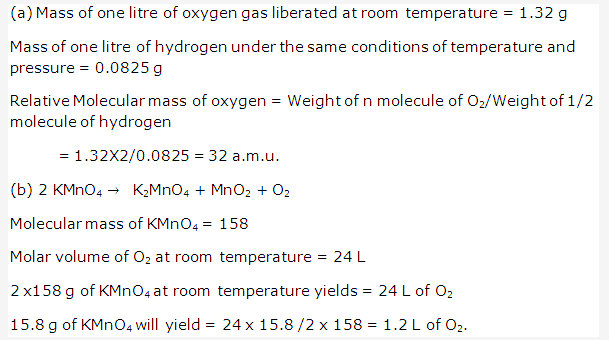
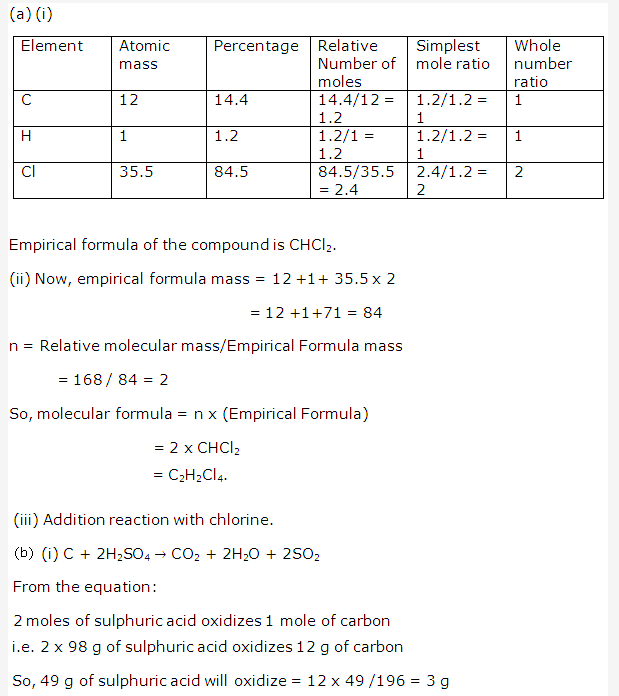
Solution 4:
- The pH of a solution is defined as the negative logarithm (base 10) of the hydronium ion concentration present in the solution.
pH =-log10 [H3O+] - The three applications of pH scale are:
- It is used to determine the acidic or basic nature of the solution.
- It is used to determine hydronium ion concentration present in the solution.
- It is used to find out neutrality of the solution.
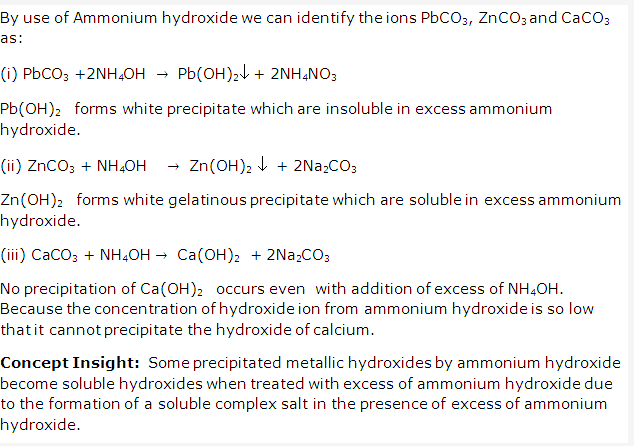
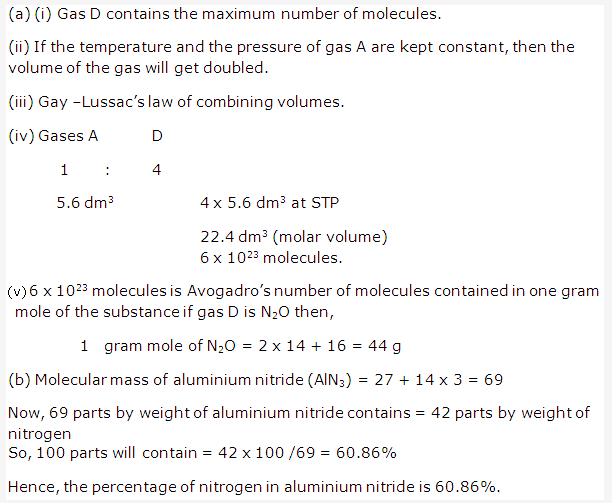
Solution 5:
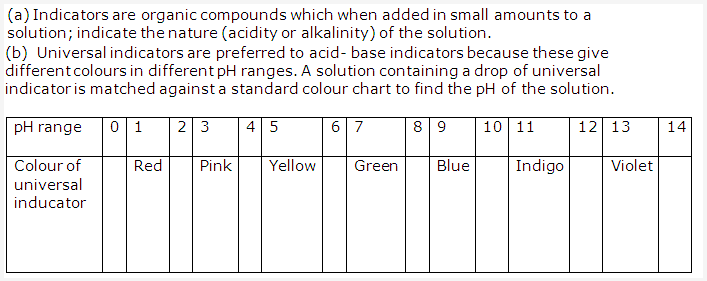
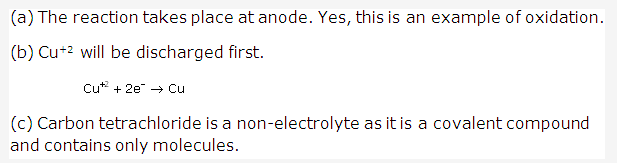
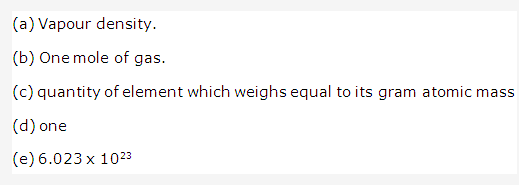
Solution 6:
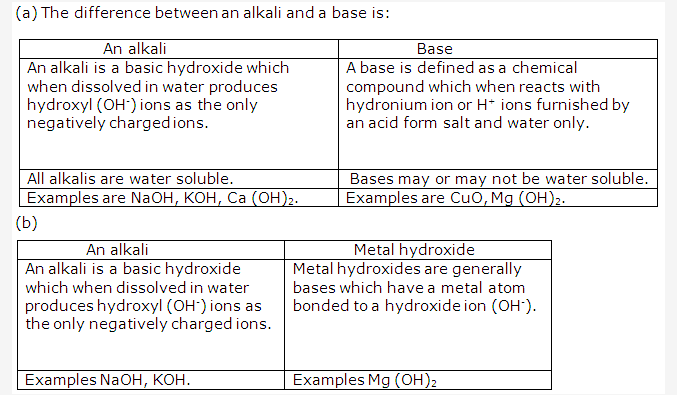
Solution 7:
- Base in solution furnishes the ions:
Hydroxide ion/ oxide ion and a metallic ion. - A weak alkali furnishes the ions:
Hydroxide ion and metallic ion and molecules of weak alkali./ - An acid in a solution furnishes the ions:
Hydronium / Hydrogen ion and a negative ion.
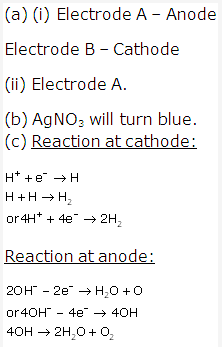
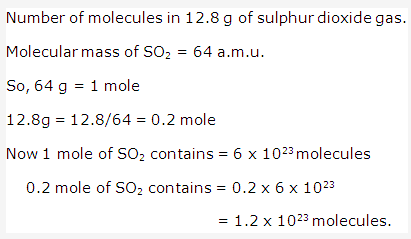
Solution 8:
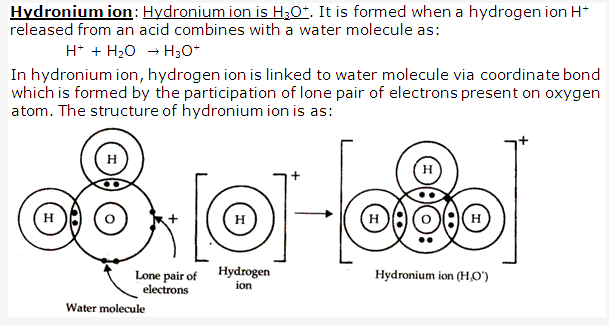
PAGE NO : 63
Solution 9:
- CaO
- NaOH
- CuO
- Cu[(OH)2]
- H2CO3
- Ferric hydroxide [Fe (OH)3].
- CuO
- NH3
Solution 10:
Anhydrous hydrogen chloride is not an acid but its aqueous solution is a strong acid because anhydrous means without water and we know that the property of acidity is shown by a substance only when it is dissolved in water or its aqueous solution is prepared.
Solution 11:
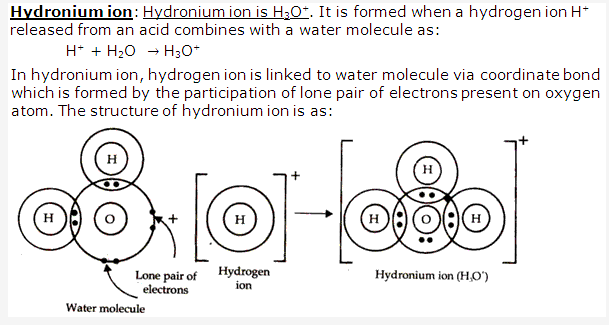
Solution 12:
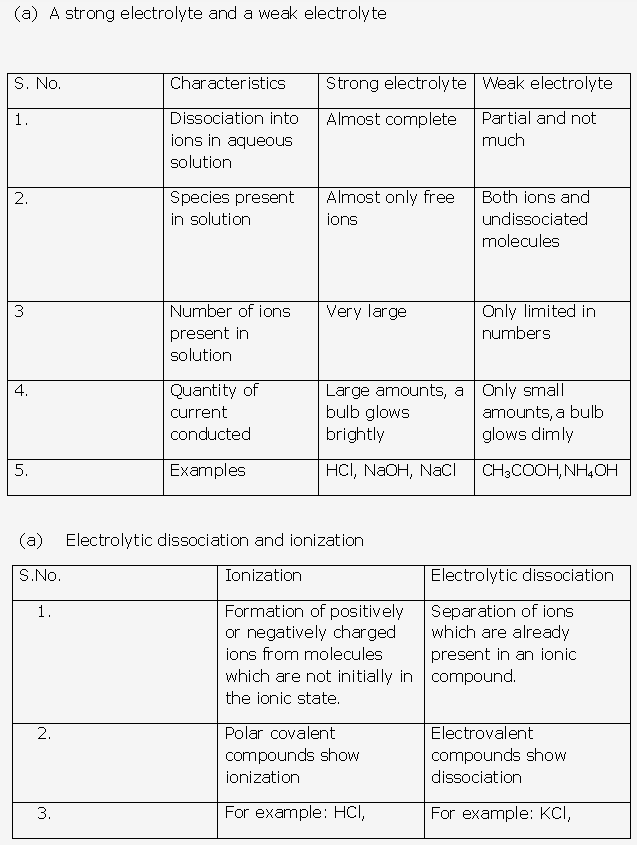
Strength of an acid measures the ease with which the acid can ionize to produce hydrogen or hydronium ions when dissolved in water. Those acids which can easily ionize to form hydrogen ions are called strong acids while those which can partially ionize to form hydrogen ions are called weak acids.
Strength of an acid depends upon many factors such as:
- Molecular structure of the acid
- The temperature
- Properties of the solvent
Solution 13:

Solution 14:
Solution B with pH value 9 will give pink colour with phenolphthalein.
Concept Insight: Bases give pink colour with phenolphthalein because a base will abstract two protons from phenolphthalein and the resulting phenolphthalein ion provides pink colour to the solution.
Solution 15:
Two indicators for identification of acid are methyl red and Thymol blue.
Solution 16:
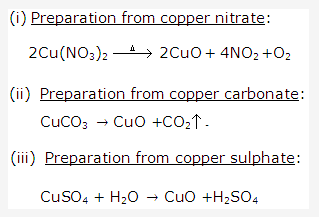
Solution 17:

Solution 18:

Solution 19:
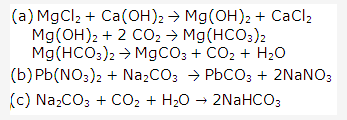
Solution 20:
- Efflorescence: It is the phenomenon by which hydrated salts on exposure to dry air, lose their water of crystallization and crumble to powder.
- Hygroscopy: It is the phenomenon by which substances absorb moisture from air, but only sufficiently so as to become wet.
- Water of crystallization: It is the fixed amount of water that is present in a crystal as an integral part of its constitution. Hydrated salts are salts having water of crystallisation.
Solution 21:
Deliquescence is the phenomenon by which certain salts absorb moisture from air, lose their water of crystallization and dissolve in it to form a saturated solution.
The substances which exhibit deliquescence are called deliquescent.
For example Caustic soda NaOH, Caustic potash KOH.
Solution 22:

Solution 23:
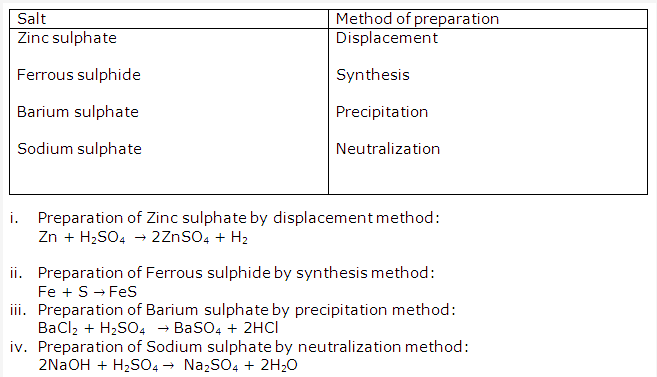
Solution 24:
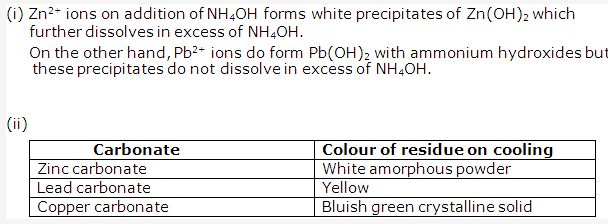
- Common salt gets wet during rainy season because the commercially available salt contains impurities, like magnesium chloride, which are deliquescent substances. These absorb moisture from atmosphere and make the table salt wet.
- (i) Na2CO4.10H2O = Washing soda
(ii) MgSO4.7H2O = Epsom salt
(iii)CuSO4.5H2O = Blue vitriol
(iv) ZnSO4.7H2O = White vitriol
PAGE NO : 64
Solution 1996-1:
- pH of a solution having pH 7 can be increased by adding a base to it such as NaOH.
- pH can be decreased by adding an acid such as HCl to it.
If a solution changes colour of litmus from red to blue, it shows that its pH is above 7.
Solution 1996-2:
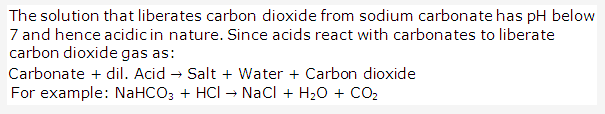
Solution 1996-3:
- Zinc sulphate = Zinc and dilute sulphuric acid
- Copper sulphate = Copper oxide and dilute sulphuric acid
- Sodium sulphate = Sodium carbonate solution and dilute sulphuric acid
- Lead sulphate = Lead carbonate and dilute sulphuric acid
Solution 1997-1:
The term acid salt means the salt formed by partial replacement of the hydrogens present in the acid by metallic or ammonium ions.
For example: NaHCO3
Solution 1997-2
- pH scale is used to express the acidic or basic nature of solution.
- pH of pure water is 7 since it does not have any impurities.
- (a) A soluble oxide of A will have pH less than the pH of pure water i.e. below 7.
(b) A solution of ‘B’ will have more pH than pure water i.e. above 7.
Solution 1997-3:
- Water of crystallization: It is the fixed amount of water that is present in a crystal as an integral pat of its constitution. Compounds having water of crystallization are called hydrous salts.
For example: Sodium carbonate Na2CO3 has 10 molecules of water present as water of crystallization Na2CO3.10H2O - Anhydrous: Hydrous salt on heating lose their water of crystallization, such salts are then called anhydrous.
For example:Na2CO3.10H2O on losing 10 molecules of water forms Na2CO3
Solution 1997-4:

Solution 1998-1:
- Water of cystallization.
- White.
- Efflorescence.
- Sodium chloride.
Solution 1998-2:
Those acids which ionize partially in aqueous solution and thus they contain ions as well as molecules of the acid. Organic acid such as CH3COOH, is a weak acid.
Solution 1998-3:
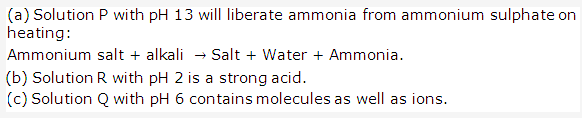
PAGE NO : 65
Solution 1998-4:
The name and formula of the acid salt which gives sodium ions and sulphate ions in solution is Sodium hydrogen sulphate NaHSO4
Solution 1999-1:

Solution 2000-1:
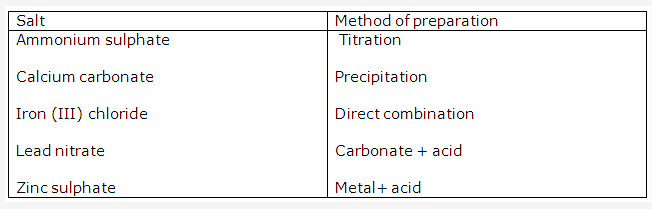
Solution 2001-1:

PAGE NO : 66
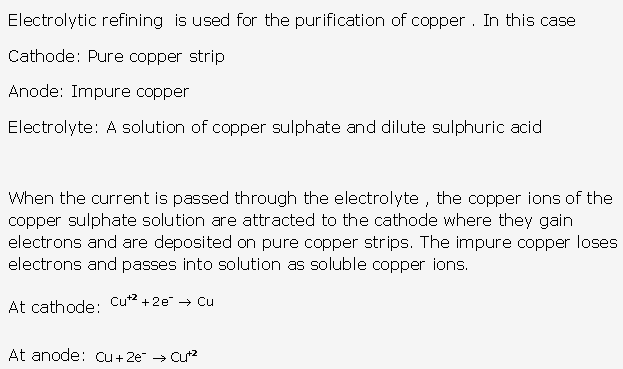
Solution 2002-1:
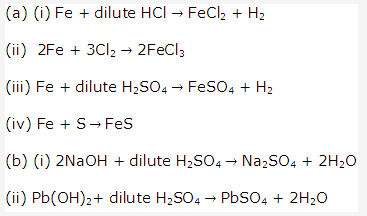
Solution 2003-1:
- Hydronium, positive.
- Acid, metal.
Solution 2003-2:
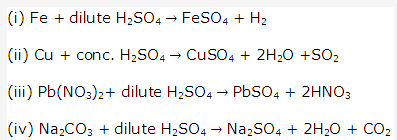
Solution 2004-1:
methods for preparation:
- Preparation of copper(II) chloride.
Action of an acid on an oxide or carbonate - Preparation of iron(III) chloride.
Direct combination - Preparation of iron (II) chloride.
Action of an acid on a metal - Preparation of lead (ii) chloride
Precipitation (double decomposition) - Preparation of sodium chloride
Neutralization of an alkali by an acid.
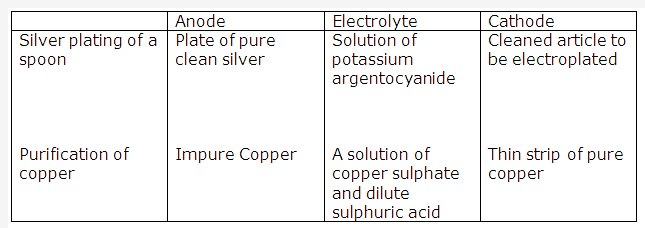
Solution 2005-1:

PAGE NO : 67
Solution 2005-2:
Positive, hydroxyl, Salt, Neutralization.
Solution 2005-3:
When neutral litmus solution is added to sodium hydrogen carbonate solution, litmus solution turns red
Solution 2006-1:
- From pink to colourless.
- From orange to pink.
- From colourless to red.
Solution 2007-1:
- Hydronium
- Hydroxide
- Salt
- Water
- Hydrogen
Solution 2007-2:
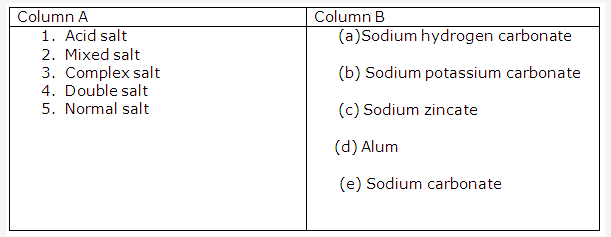
Solution 2008-1:
- Complex salt.
- Alkali.
Solution 2009-1:
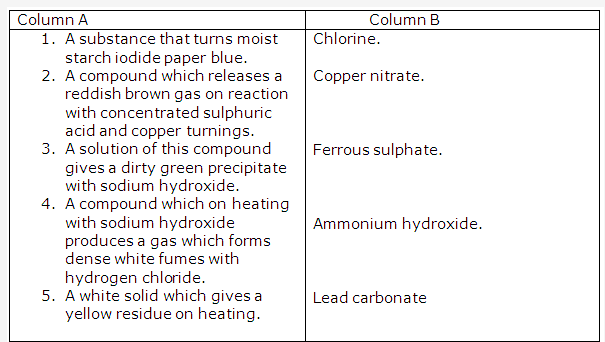
Acidified potassium dichromate paper
PAGE NO : 68
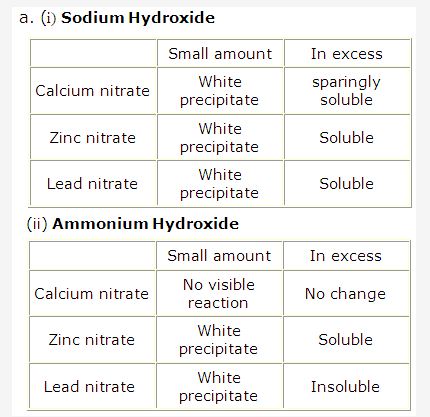
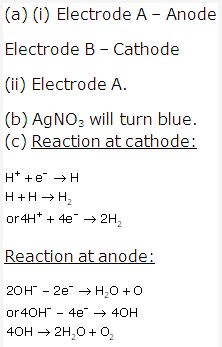
Solution 2009-2:
- Solution B.
- Solution A.
- Solution B
- Solution of ammonium hydroxide NH4OH is a weak alkali.
Solution 2009-3: