What is alcohol in chemistry?
What is the functional group in an alcohol?
Alcohols
- Alcohols are non-hydrocarbons.
- It is a homologous series of organic compounds containing not only the elements carbon and hydrogen, but also oxygen.
- Alcohols contain the hydroxyl group, (– O – H) as their functional group. The hydroxyl group is the part of the alcohol molecule which determines its chemical properties.
- This group is covalently bonded to a carbon atom as shown below.

- It should not be confused with the hydroxide ion, OH–. There is no hydroxide ion in an alcohol molecule. Hence, alcohols do not show alkaline properties. In fact, alcohols are neutral compounds.
- Alcohols may be considered to be derived from an alkane by the replacement of one hydrogen atom by the hydroxyl group.
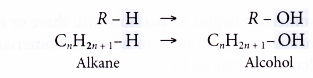
Hence, the general formula of the alcohol family is CnH2n+1OH, where n = 1, 2, 3,…
How do we name alcohols?
Naming alcohols
- The IUPAC name for the alcohol family is alkanol.
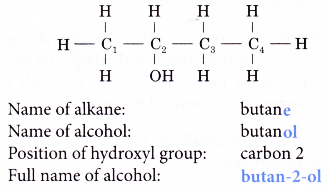
- Naming a straight-chain alcohol:
Step 1: Obtain the name of the alkane with the same number of carbon atoms as the alcohol.
Step 2: Replace the ending -e from the name of the alkane with –ol.
Step 3: A number is placed to in front of the –ol to indicate which carbon atom the hydroxyl group is attached to.
For example:
Isomers of alcohols
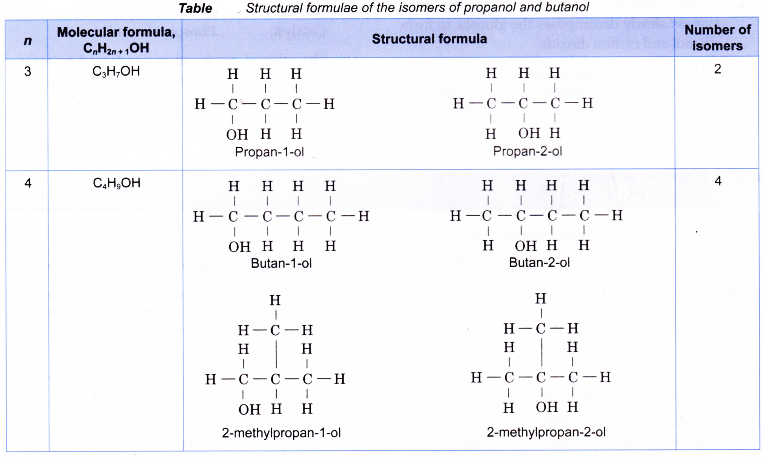
- Isomerism begins in alcohols with three or more carbon atoms. Similar to alkenes, isomerism in alcohols is caused by
(a) the branching of the carbon chain
(b) the different locations of the hydroxyl group - Structures I and II are two examples of how branching of the carbon chain occurs in alcohol molecules.
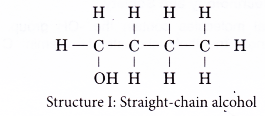
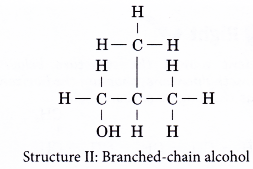
- The positions of the hydroxyl group, -OH are different in structures III and IV even though they have the same carbon skeleton.
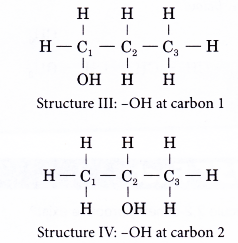
- The following example shows how an isomer of an alcohol can be named.
Naming alcohols example:
Name the structure shown below.
Solution:
Step 1: Find the longest continuous carbon chain containing the hydroxyl group.
Step 2: Name this longest chain by substituting the ending -ol for the -e of the corresponding alkane.
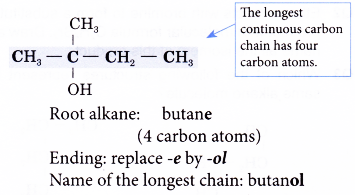
Step 3: Number the carbon atoms in this longest chain beginning at the end nearer to the hydroxyl group. This will ensure that the hydroxyl group always receives the lowest possible number.
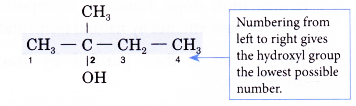
Step 4: Identify the position of the hydroxyl group by writing the number of the carbon atom to which it is attached in front of the ending -0l.
Note: The position of the hydroxyl group needs to be indicated only for chains of three or more carbon atoms.
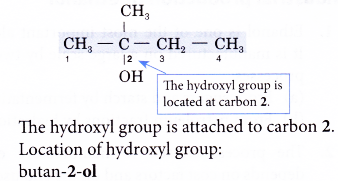
Step 5: Locate and name the attached alkyl group.
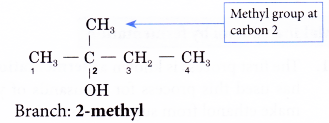
Step 6: Complete the name for the alcohol molecule by combining the three component parts together. Write the name as a single word.
Name of the molecule: 2-methylbutan-2-ol
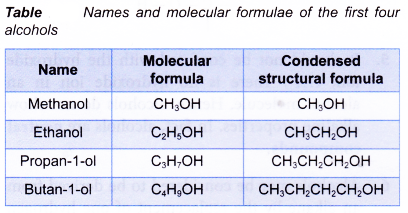
Table shows the structural formulae of the isomers of propanol and butanol.
The first two members of the alcohol family, methanol and ethanol, do not have isomers. Their structural formulae are as shown below.
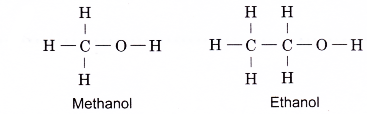
People also ask
- What are carbon compounds?
- Chemical Properties of Carbon Compounds
- How are alkanes formed?
- What is an alkene in chemistry?
- What is an isomerism?
- How are carboxylic acids formed?
- How esters are formed?
- What are fats and oils?
- How palm oil is extracted?
- Order in Homologous Series
- What is the monomer of natural rubber?
- Which acid is used for coagulating rubber from latex?
- Classification of Hydrocarbons
- What is the homologous series of hydrocarbons?
- Properties and Uses of Ethanol
- Properties and Uses of Ethanoic Acid
How is ethyl alcohol made?
Industrial production of ethanol
- Ethanol is one of the most important alcohols. It is manufactured on a large scale by two main processes:
(a) From sugars and starch by fermentation
(b) From petroleum fractions by hydration - The process chosen to manufacture ethanol depends on cost factors and on the end use of the product.
How do you make ethanol by fermentation?
Making ethanol by fermentation
- The first process is known as fermentation. Man has used this process for thousands of years to make ethanol from sugars and starch.
- Yeast is added to sugar or starch and left in a warm place for several days in the absence of air. The fermentation process is anaerobic, which means it takes place in the absence of oxygen.
- Yeast releases biological enzymes, which break down the sugars and starch into glucose.
- In the fermentation process, the enzyme called zymase slowly decomposes the glucose to form ethanol and carbon dioxide.


- When the concentration of ethanol formed reaches about 15%, yeast is killed off and the fermentation stops. Hence, it is impossible to produce pure ethanol by natural fermentation.
- The ethanol is then purified by fractional distillation.
Making ethanol by hydration
- The second process is called catalytic hydration, the direct hydration of ethene.
- Ethene is obtained from the cracking of petroleum fractions.
- An addition reaction combines ethene with water to produce ethanol.

- The ethanol produced is condensed to form a liquid and the unreacted ethene is recycled.
Preparation of ethanol in the laboratory experiment
Aim: To prepare samples of ethanol.
Materials: Glucose, yeast, limewater, distilled water, filter paper, mashed pineapple, cooked potatoes, tap water.
Apparatus: 250 cm3 conical flask, 150 cm3 conical flask, 500 cm3 beaker, 50 cm3 measuring cylinder, distillationflask, stopper with delivery tube, stopper with one hole, thermometer, Liebig condenser, fractionating column, retort stands and clamps, tripod stand, wire gauze, Bunsen burner, rubber tubing, filter funnel, boiling tube.
Procedure:
- About 20 g of glucose is dissolved in 150 cm3 of distilled water contained in a clean conical flask.
- About 10 g of yeast is added to the mixture and the mixture is shaken well.
- The conical flask is closed with a stopper connected with a delivery tube. The other end of the delivery tube is dipped into limewater in a boiling tube as shown in Figure (a).
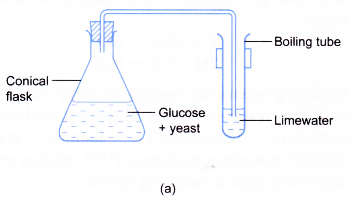
- The apparatus is left in a warm place (about 37°C) for about a week.
- From time to time, any changes taking place are observed.
- After about a week, the contents of the conical flask are filtered. The filtrate is poured into a distillation flask.
- The apparatus for distillation as shown in Figure (b) is set up.
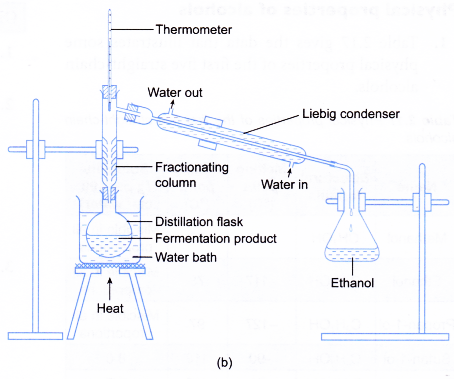
- The filtrate is heated in a water bath and the ethanol that boils over at 78 to 80°C is collected.
- The colour and smell of the distillate collected is examined.
- Steps 1 to 9 are repeated using mashed pineapple and cooked potatoes consecutively to replace glucose.
Observations:
- A colourless gas is liberated during the fermentation process. The gas turns limewater milky.
- A colourless liquid is obtained during the fractional distillation.
Discussion:
- The enzyme from yeast slowly decomposes glucose in the absence of oxygen to form ethanol and carbon dioxide.
The carbon dioxide released turns limewater milky.
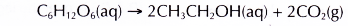
- Fermentation occurs efficiently at a temperature of 37°C when the activity of the enzyme is at its peak.
- Water and ethanol are two miscible liquids with different boiling points. Hence, both liquids have to be separated by fractional distillation.
Conclusion:
Ethanol can be obtained by the fermentation of glucose found in sugars or carbohydrates.
What are the physical properties of alcohol?
Physical properties of alcohols
- Table gives the data that illustrates some physical properties of the first five straight-chain alcohols.
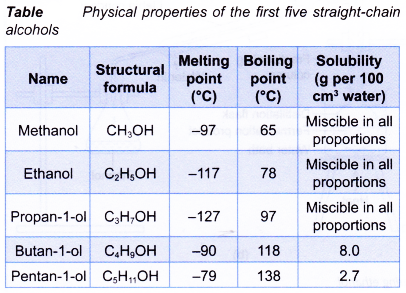
- Simple alcohols are liquids at room conditions.
- Simple alcohols are very soluble in water. They have infinite solubility in water.
- Physical properties of ethanol:
(a) It is a liquid at room conditions.
(b) It is colourless.
(c) It has a sharp smell.
(d) It is completely miscible with water.
(e) It has a low boiling point (78°C).
(f) It is highly volatile.
Chemical properties of ethanol
Ethanol has a set of reactions determined by its functional group, the hydroxyl group.
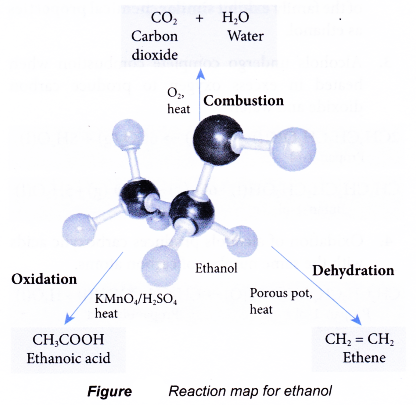
Combustion:
- Ethanol is a very flammable substance. It readily undergoes combustion when heated in oxygen. Ethanol burns with a non-smoky blue flame. Complete combustion of ethanol produces carbon dioxide and water, as shown in the equation below.
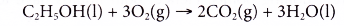
- Besides carbon dioxide and water, the combustion of ethanol releases large amount of heat. This makes ethanol suitable for use as a fuel. It is described as a clean fuel because it does not release pollutants into the atmosphere.
Oxidation:
- Oxidation reaction occurs when a substance combines with oxygen.
- Combustion is an example of an oxidation reaction. In the combustion of ethanol, carbon combines with oxygen to form carbon dioxide and hydrogen combines with oxygen to form water.
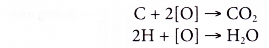
- Oxidising agents are needed to carry out the oxidation reactions. In the laboratory, acidified potassium dichromate(VI) solution and acidified potassium manganate(VII) solution are commonly used as oxidising agents.
- Alcohols can be easily oxidised to carboxylic acids, a family of organic compounds which has the -COOH group.
- When acidified potassium dichromate(VI) solution is added to ethanol and the mixture is warmed, it turns from orange to green. This shows that it has oxidised the ethanol to form ethanoic acid.
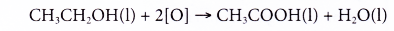
- When acidified potassium manganate(VII) solution is used instead, it is decolourised. Purple potassium manganate(VII) is changed to colourless.
Dehydration:
- Dehydration involves the removal of water from a compound. In the dehydration of alcohols, a molecule of water is eliminated from each alcohol molecule. The alcohol molecule is changed into an alkene molecule. Hence, this reaction can be used to make alkenes from alcohols.
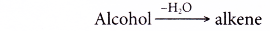
- The dehydration of ethanol produdes ethene. The elimination of a molecule of water results in the formation of a carbon-carbon double bond.
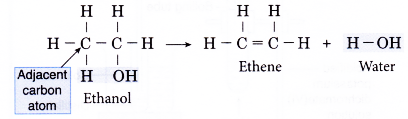
- Notice that the hydroxyl group is removed together with a hydrogen atom from an adjacent carbon atom.
- The ethene produced can be detected by the following tests:
(a) It decolourises red-brown bromine water.
(b) It decolourises purple acidified potassium manganate(VII) solution. - There are two methods to carry out a dehydration of ethanol in the laboratory.
(a) Ethanol vapour is passed over a heated catalyst such as unglazed porcelain chips, porous pot, pumice stone or aluminium oxide.
(b) Ethanol is heated under reflux at 170°C with excess concentrated sulphuric acid.
Chemical properties of ethanol experiment
Aim: To investigate the chemical properties of ethanol.
Materials: Ethanol, potassium dichromate(VI) solution, potassium manganate(VII) solution, concentrated sulphuric acid, bromine water, blue litmus paper, glass wool, ice, unglazed porcelain chips, wooden splint, water.
Apparatus: Evaporating dish, test tubes, boiling tube, stopper with delivery tube, test tube holder, beaker, retort stand and clamp, Bunsen burner, 10 cm3 measuring cylinder, dropper, forceps.
A. Combustion of ethanol
Procedure:
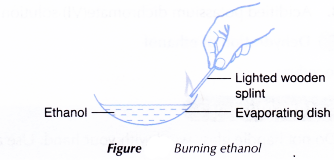
- About 2 cm3 of ethanol is poured into an evaporating dish.
- A lighted wooden splint is used to ignite the ethanol.
- The combustibility of ethanol is noted. The colour and sootiness of the resulting flame is also noted.
Observations:
| Test | Observation |
| Combustibility | Catches fire readily |
| Colour of flame | Blue |
| Sootiness of flame | Non-sooty |
Discussion:
- Ethanol burns readily in air to produce carbon dioxide, water and heat.
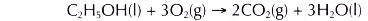
- Ethanol can be used as a fuel for cars because it burns cleanly and gives out large quantities of heat.
B. Oxidation of ethanol
Procedure:
- About 10 cm3 of potassium dichromate(VI) solution is poured into a boiling tube.
- A dropper is used to add about 10 drops of concentrated sulphuric acid into the boiling tube.
- About 3 cm3 of ethanol is then added to the mixture.
- The apparatus is set up as shown in Figure.
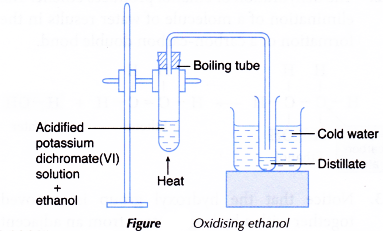
- The boiling tube is heated carefully with a gentle flame until the mixture boils. Any change in colour of the mixture is noted.
- The distillate is collected in a test tube immersed in cold water.
- The colour and smell of the distillate are noted.
- The distillate is tested with a piece of moist blue litmus paper.
- The change to the blue litmus paper is recorded.
Observations:
- The acidified potassium dichromate(VI) solution changes from orange to green.
- Observations on distillate:
Test on distillate Observation Colour Colourless liquid Smell Smell of vinegar Effect on blue litmus paper Blue litmus paper turns red.
Discussion:
- Ethanol is oxidised to ethanoic acid.
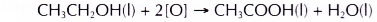
- Ethanoic acid is a colourless liquid with a smell of vinegar and turns blue litmus paper red.
- Acidified potassium dichromate(VI) solution is the oxidising agent.
C. Dehydration of ethanol
Procedure:
- Some glass wool is placed in a boiling tube.
- Using a dropper, some ethanol is added into the boiling tube to wet the glass wool.
- The boiling tube is clamped horizontally and unglazed porcelain chips are placed in the mid-section of the boiling tube.
- The boiling tube is closed with a stopper fitted with a delivery tube. The other end of the delivery tube is placed under an inverted test tube filled with water.
- The apparatus is set up as shown in Figure.
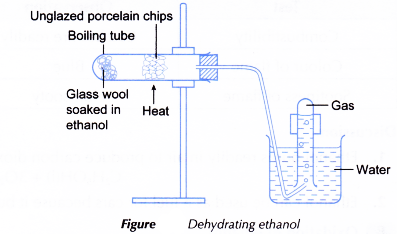
- The unglazed porcelain chips are heated strongly. When the porcelain chips are hot, the flame is shifted to heat gently the glass wool to vaporise the ethanol.
- Heating is continued to produce enough gas to fill two test tubes.
- The following tests are carried out on the gas collected in the two test tubes.
(a) About 1 cm3 of bromine water is added to the first test tube and the mixture is shaken well.
(b) About 1 cm3 of acidified potassium manganate(VII) solution is added to the second test tube and the mixture is shaken well. - All observations are recorded.
Observations:
| Reagent | Observation |
| Bromine water | Red-brown bromine water is decolourised. |
| Acidified potassium manganate(VII) solution | Purple potassium manganate(VII) solution is decolourised. |
Discussion:
- Dehydration of ethanol produces ethene.
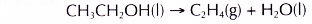
- Ethene is an unsaturated hydrocarbon. It decolourises both bromine water and acidified potassium manganate(VII) solution.
Conclusion:
The chemical reactions of ethanol are:
(a) Combustion to form carbon dioxide and water
(b) Oxidation to produce ethanoic acid
(c) Dehydration to produce ethene
How do the other alcohols react?
- Ethanol is a member of the alcohol homologous series. All the other members of this series have the same functional group as ethanol, which is the hydroxyl group, -OH.
- It is the hydroxyl group which gives ethanol its chemical properties. Hence, the other members of the family exhibit similar chemical properties as ethanol.
- Alcohols undergo complete combustion when heated in excess oxygen to produce carbon dioxide and water.
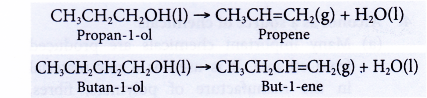
- Oxidation of alcohols produces carboxylic acids with the same number of carbon atoms.
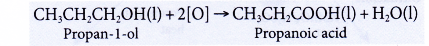
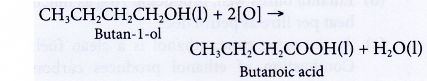
- Dehydration of alcohols produces the corresponding alkenes.
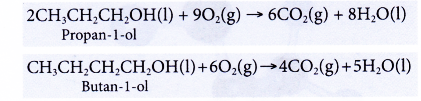
What are the uses of alcohol?
Uses of alcohols
1. Alcohols have many properties which make them suitable for producing many things that we use in everyday life. Figure summarises the uses of ethanol.
2. Alcohol as a solvent
- Ethanol is a colourless, volatile liquid with a low boiling point (78°C). Ethanol is relatively safe, miscible with water and is able to dissolve many organic molecules which do not dissolve in water.
- It is a valuable solvent in the laboratory and industry. As a solvent, it is used in perfumes, cosmetics and toiletries, and also in thinners for lacquers, varnishes and printing inks. Being volatile, the alcohols evaporate easily and leave the solute behind.
- Other alcohols such as methanol and propanol are also used as solvents for paints, lacquers, shellacs and industrial cleaners for cleaning compact discs and record/playback heads in tape machines.
3. Alcohol as a fuel
- The recent rise in the price of crude oil has forced many countries which have to import oil to find alternative fuels. One such alternative fuel is ethanol. A mixture of petrol with 10 – 20% ethanol is called gasohol.
- Ethanol burns well, producing 70% as much heat per litre as petrol does.
- At the same time, ethanol is a clean fuel. Combustion of ethanol produces carbon dioxide and water.
- Methanol is used as a fuel for racing cars.
4. Alcohol as a source of chemicals
- Many important chemicals are produced from ethanol. It is used as a raw material in the manufacture of polymers, fibres, explosives and plastics.
- Ethanol is oxidised to ethanoic acid for making vinegar.
- Methanol is oxidised to methanal which is used as a chemical feedstock to make formaldehyde resins.
5. Alcohol as a source of medicinal products
- Ethanol is also used in the making of many pharmaceutical products.
- Ethanol is used as a solvent in the preparation of tinctures (solutions of iodine in ethanol) and cough syrups.
- A 70% by volume of solutions of ethanol or propan-2-ol are excellent antiseptics for skin disinfection.
- The solution of propan-2-ol is commonly known as rubbing alcohol. When it is rubbed onto the skin, the rapid evaporation rate of the alcohol creates a dramatic cooling effect. Hence, it is suitable for use to bring down high fever (body temperature).
Alcohol – misuse and abuse
- The human race has been consuming ethanol in the form of alcoholic drinks for centuries, although the dangers of its abuse have long been known.
- Doctors have also repeatedly reminded users about the harmful effects of ethanol.
- Ethanol is a central nervous system depressant drug. It slows down both physical and mental activity. This causes the person to feel high and to lose shyness.
- The person in this state of mind is no longer in control of his actions. He loses both physical and mental control. Driving a car by such a person can cause fatal accidents.
- Ethanol is also an addictive drug. Long-term excessive use of ethanol is known to cause chronic liver disease (cirrhosis) and brain damage, eventually leading to death.
- Doctors have also established links between certain birth defects and the consumption of alcohol by mothers during pregnancy.
Reaction map for ethanol