Plus Two Physics Chapter Wise Previous Questions Chapter 13 Nuclei is part of Kerala Plus Two Physics Chapter Wise Previous Questions and Answers Kerala. Here we have given Plus Two Physics Chapter Wise Questions and Answers Chapter 13 Nuclei.
Kerala Plus Two Physics Chapter Wise Previous Questions and Answers Chapter 13 Nuclei
Question 1.
After 1 hour, 1/8 of the initial mass of a certain radioactive isotope remains undecayed. The half-life of the isotope is (March – 2009)
a) 20 minutes
b) 30 minutes
c) 45 minutes
d) 8 minutes
Answer:
1/8 of the initial mass of certain radioactive isotope remains undecayed means 3 half lives have elapsed. Therefore 3 half lives = 60 minutes. Hence half life = 20 minutes.
Question 2.
a) How many electrons and neutrons are there in AXZ? (March – 2009)
b) Can an electron reside inside the nucleus?
c) Can an electron be emitted from the nucleus? Explain.
Answer:
a) Number of electrons / protons = z
Number of neutrons = A – Z
b) No
c) Yes Sometimes an neutron inside the nucleus converts into a proton, electron and antineutrino.
Question 3.
The half-life of Radon is 3.8 days. (March – 2010)
a) Define half-life.
b) Calculate how much of 15 mg of Radon will remain after 14.2 days.
Answer:
a) Half-life of radioactive sample is defined as the time taken it takes for the number of radioactive nuclei in the sample to reduce to half.
b) Number of half-lives
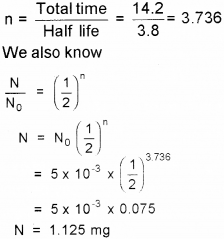
Question 4.
Nuclear fission and nuclear fusion represent two types of nuclear reactions. Then (Say – 2010)
a) Write down one similarity and one dissimilarity between nuclear fission and nuclear fusion.
b) Fora nuclear reaction, along with masses of nuclei P, Q, R and S taking part in it is given below
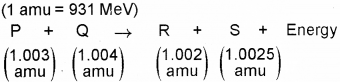
How much energy is released by the reaction (in MeV)?
Answer:
a) Similarity
1) Both produce large amount of energy than chemical reaction.
2) Structure of nucleus changes.
Dissimilarity
1) Fission can be controlled but fusion can’t be controlled.
2) Fission produces radiation, fusion doesn’t produce radiation.
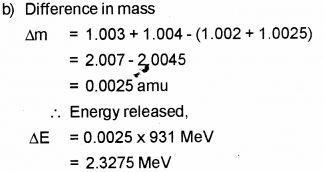
Question 5.
A radioactive substance decays to 1/32 of its initial value in 25 days. Calculate its half life. (Say – 2011)
Answer:
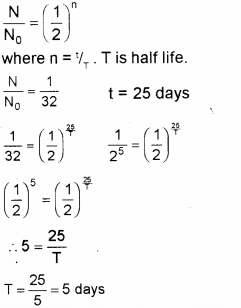
Question 6.
a) In the following nuclear fission reaction, N is the number of neutrons released. What is the value of N? (March – 2012)
![]()
b) Complete the following nuclear reaction equation.
![]()
c) Two nuclei have mass numbers in the ratio 1:8. What is the ratio of their nuclear radii?
Answer:
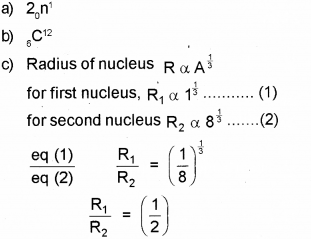
Question 7.
Nucleus of an atom consists of protons and neutrons closely packed into a small volume. (Say – 2012)
a) How will relate the binding energy and stability of a nucleus?
b) Explain how energy is released in a fission process.
c) Nuclear fusion is the source of ene rgyin stars.
Write down the reactions involved in a proton-proton cycle.
Answer:
a) When binding energy per nucleon of a nucleus increases, its stability also increases.
b) In nuclear fission, certain mass disappears. ie sum of the masses of final products is found to be slightly less than the sum of the masses of the reactant components. This difference in mass is converted in to energy in nuclear fission.
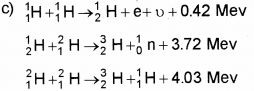
Question 8.
In a nuclear reactor the chain reaction is carried out under controlled conditions. (March – 2013)
a) Name the material that is used as control rods in a nuclear reactor.
b) Average energy of a neutron produced in fission of \(_{ 92 }^{ 235 }{ U }\) nucleus is ……………………….
c) Write down the reactions involved in the con version of \(_{ 92 }^{ 235 }{ U }\) to \(_{ 92 }^{ 235 }{ Pu }\) to in a nuclear reactor.
Answer:
a) Cadmium
b) 2 Mev

Question 9.
Spontaneous a’nd continuous disintegration of a nucleus of a heavy element with the emissio of cer tain type os radiation is known as radioactivity. (Say – 2013)
a) The radioactive isotope ‘D’ decays according to the sequence
![]()
If the mass no. and atomic no. of D2 are 176 and 71 respectively, what is the-
i) mass number
ii) atomic number of D?
b) State radioactive decay law.
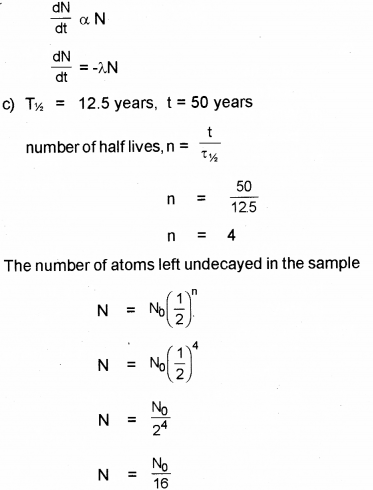
d) Tritium has a half-life of 12.5 years. What fraction of a sample of pure tritium will remain undecayed after 50 years?
Answer:
![]()
i) Mass numbe of D is 180
ii) Atomic number of D is 72
b) According to law of radioactive decay, the number of nuclei undergoing decay per unit time is proportional to number of nuclei in the sample at that time.
Question 10.
Energy generation in stars is due to nuclear fusion. (March – 2014)
a) How a nuclear fusion is occurred?
b) The energy released in nuclear fission process with uranium is of the order of ……………….
c) Three types of radio active decay occur in nature. Briefly describe them.
d) State the law of radio active decay.
Answer:
a) Two light nuclei combine together to form heavy nucleus at high temperature. This is called nuclear fusion.
b) 200MeV
c) Alpha Decay (α decay)
In α decay, mass number is reduced by 4 units and atomic number is reduced by 2 units.
Beta-decay (β-decay)
There are two types of β decay (i) β– decay (ii) β– decay
i) β+ decay
In β+ decay atomic number is reduced by 1 unit. But mass number remains unchanged.
ii) β decay
In β– decay, atomic number is increased by 1 unit. But mass number does not change.
Gamma Decay
The excited nucleus comes back to ground state by emitting gamma rays.
d) Refer to Question 1b, Say 2013
Question 11.
Radio activity is an accidental discovery by Henry Becqueral. (Say – 2014)
a) Catagorize the following statements related to radioactivity as true or false.
i) All elements present in nature show radioactivity.
ii) Rate of decay of all radio active elements can be characterized by their half life period.
b) A certain radio active element has a half life of 10 days. How long it will take for 75% of its atoms originally present to disintegrate?
Answer:
a) i) False
ii) True
b) 75% of atoms are decayed. Hence N = 0.25N0 We know
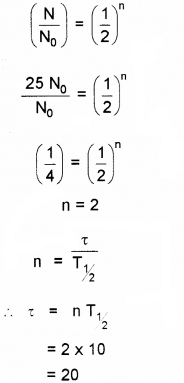
Question 12.
Nuclear fusion reaction is responsible for energy generation in stars.
a) What is the principle behind a nuclear reactor
i) fission
ii) fusion
iii) controlled fusion
b) Which of the following helps to slow down fast neutrons in a reactor
i) safety rods
ii) moderators
c) In the following nuclear reaction what is the element X?
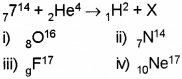
d) The mass of an atom is expressed in
i) Kg
ii) g
iii) u
iv) carats
Answer:
a) fission
b) moderators
c) 8O16
d) u
Question 13.
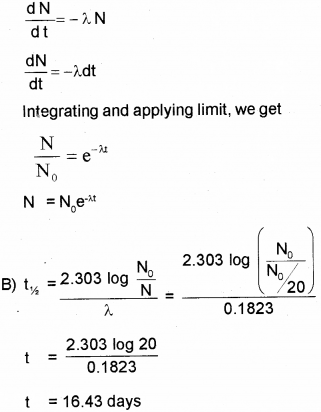
A) Obtain an expression for the number of radioactive nuclei present at any instant in terms of the decay constant and initial number of nuclei. (March – 2015)
B) The half life of radioactive radon is 3.8 days. Find the time during which 1/20 of radon sample will remain undecayed.
Answer:
A) According to Law of Radioactive decay.
Question 14.
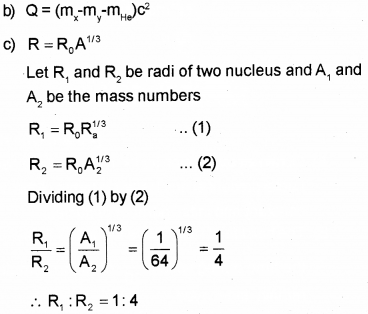
a) What do you mean by Q value of a nuclear reaction? (March – 2016)
b) Write down the expression for Q value in the case of decay.
c) Two nuclei have mass numbers in the ratio 1:64. What is the ratio of their nuclear radii?
Answer:
a) Q-value : Q value is the energy released in nuclear reaction. The Q value or disintegration energy of a decay can be defined as the difference between the initial mass energy and final mass-energy of decay products. The Q value of a decay is expressed as
Question 15.
The force exists between the nucleons in a nucleus is called nuclear force. (Say – 2016)
a) The nuclear force betwen two protons, two neutrons and between a proton and neutron is denoted by fpp, fnn and Pn respectively, then
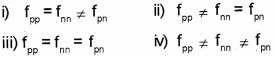
b) What is the meaning of mass defect?
c) Calculate the energy released in the nuclear re action shown below:
1H2 + 1H2 → 2H4 + Energy
mass of (1H2)= 2.014102 u
mass of (2H4) = 4.0026 u
I a.m.u = 931 MeV
Answer:
a) iii) fpp = fnn = Pn
b) The mass defect is the difference in the mass of nucleus and total mass of the constituent nucleons.
c) Toal mass of reactants (1H2 + 1H2)
= 2.014102+2.014102=4.028204 amu
Total mass of product = 4.0026 amu
Mass defect = 4.0026 – 4.028204 = 0.25604 amu
Energy released = 0.25604 x 931 Mev
= 23.84 Mev
Question 16.
a) Define half life period of a radioactive nucleus. Write down the relation connecting half life period and mean life. (March – 2017)
b) Define 1 amu. Calculate its energy equivalent in MeV.
Answer:
a) Half life is the time taken by radio nuclide to reduce half its initial value.
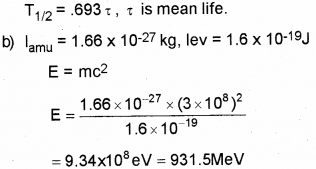
Question 17.
a) A and B are two isotopes having mass numbers 14 and 16 respectively. If the atomic number of A is 7, then how many neutrons will be there in B? (Say – 2017)
b) The half-life period of a radioactive material is 5 hours. In how much time 15/16 of the material will decay?
Answer:
a) Number of neutron = mass number- atomic number = 16 – 7 = 9
b) In this case, 15/16 of the mass is decayed. Which means that of the mass remain undecayed. Number of half life required for this can be found using this formula
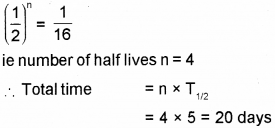
We hope the Kerala Plus Two Chemistry Chapter Wise Questions and Answers Chapter 13 Nuclei help you. If you have any query regarding Kerala Plus Two Chemistry Chapter Wise Questions and Answers Chapter 13 Nuclei, drop a comment below and we will get back to you at the earliest.