Plus Two Physics Chapter Wise Previous Questions Chapter 12 Atoms is part of Kerala Plus Two Physics Chapter Wise Previous Questions and Answers Kerala. Here we have given Plus Two Physics Chapter Wise Questions and Answers Chapter 12 Atoms.
Kerala Plus Two Physics Chapter Wise Previous Questions Chapter 12 Atoms
Question 1.
The total energy of an electron in the ground state of a hydrogen atom is -13.6 eV. (March – 2010)
a) What do you mean by ground state of a hydrogen atom ?
b) The excitation energy required to raise the elec-tron in the first excited state of hydrogen atom is ……………. eV.
Answer:
a) The most stable state of hydrogen atom having energy -13.6 eV is called ground state.
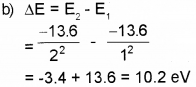
Question 2.
If the wavelength ‘λ’ of spectral lines emitted by hydrogen atom is generally expressed as (Say – 2010)
![]()
Where R : Rydberg Constant and ‘n’ and ‘m’ are integers. From this,
a) Write down the expression for Balmer series of spectral lines.
b) Find out the shortest wavelength of spectral line emitted in Balmerseries.
Answer:
a) Far Balmer Series,
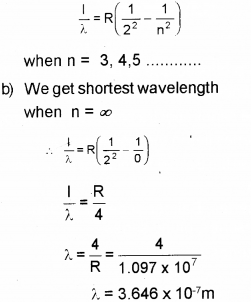
Question 3.
Bohr proposed a new model of atom to overcome a problem of Rutherford’s atom model. (March – 2011)
a) Which specific problem of the Rutherford model was attempted to be solved by Bohr model?
b) What are the basic postulates of Bohr model?
c) The radius of the inner most electron orbit of a hydrogen atom is 5.3 x 10-11m. What are the radii of the orbit n=2 and n = 3?
Answer:
a) Stability of atom
b) Bohr combined classical and early quantum con-cepts and gave his theory in the form of three postulates.
- Electrons revolve round the positively charged nucleus in circular orbits.
- The electron which remains in a privileged path cannot radiate its energy.
- The orbital angular momentum of the electron is an integral multiple of h/2π.
- Emission or Absorption of energy takes place when an electron jumps from one orbit to another.
c) We know radius, r α n2 for first orbit
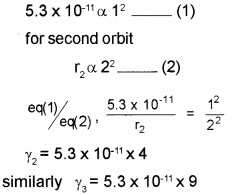
Question 4.
Spectra are produced due to electronic transitions from higher energy level to lower energy level. (Say – 2011)
a) Name the series spectra produced by hydrogen.
b) Which series lies in the visible region?
c) Write down the Balmer formula for the wavelength of Ha line.
Answer:
a) Lyman series, Balmer senes, Paschen series, Brackett series, pfund series.
b) Balmer series
![]()
Question 5.
Niels Bohr explaiq^d hydrogen spectrum based on quantum ideas. (March – 2012)
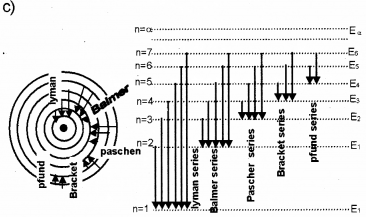
a) Drawthe energy level diagram of hydrogen atom.
b) Name the different series of lines observed in hydrogen spectrum.
c) Show the transitions between energy levels producing the different series.
Answer:
a) n = ∞ …………………………
n = 8 …………………………
n = 7 …………………………
n = 6 …………………………
n = 5 …………………………
n = 4 …………………………
n = 3 …………………………
n = 2 …………………………
n = 1 …………………………
b) Lyman series, Balmer series, Paschen series, Bracket series, P-fund series.
Question 6.
In 1913 Neils Bohr proposed a successful atom model by combining the classical and quantum concepts. (Say – 2012)
a) Based on Bohr atom model explain the line spectra of hydrogen atom.
b) Calculate the radius of third Bohr orbit of hydrogen atom and the energy of electrons ¡n that orbit.
h = 6.625 x 10-34 JS,
εe = 8.85 x 1012 FM-1,
e = 1.6 x 10-19 C,
me = 9.1 x 10-31 Kg)
Answer:
a) According to the third postulate of Bohr’s model, when an atom makes a transition from a higher energy state (n1) to lower energy state (n1) a photon of energy htj1is emittedle. ie. huif = Eni – Ent depending upon the transition between energy levels there are different types of spectral senes for hydragen atom
- Lyman series
- Balmer series
- paschan series
- Bracket series
- P-fund series
b) radius of hydragen atom,
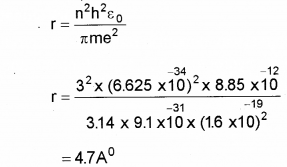
Question 7.
Rutherford’s atom model is based on the classical concept that electrons are revolving around a central positive nucleus. (March – 2013)
a) Mention the drawback of Rutherford atom model and how it rectified in Bohr’s atom model?
b) From Bohr’s theory obtain the de Broglie wave length of an electron orbiting around the nucleus.
c) Give the statement fo Heisenbergs uncertainty principle and express ¡t mathematically.
Answer:
a) Rutherford’s model of atom could not explain the stability of an atom.
Bohr’s model of atom could explain the stability of an atom. According to Bohr, electron cari revolve only in stationary orbits.
b) According to Bohr’s postulate
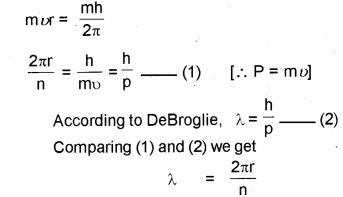
c) According to Heisenbergs uncertainty principle, it is not possible to measure both the position and momentum of an electron (or any other particle) at the sam exactly.
ie, Δx Δp = h/2π
Where Δx → uncertainty in the position.
Δx → uncertainty in the momentum.
Question 8.
When a vapour is excited at low pressure by passing an electric current through it, a spectrum is obtained. (March – 2014)
a) Draw a spectral series of emission lines in hydrogen.
b) Name the different series of hydrogen atom.
c) In which region Lyman series is located.
Answer:
a) Refer Q. No. ic, March 2012
b) Lyman series, Balmer series, Paschen series, Bracket series, Pfund series.
c) UV region
Question 9.
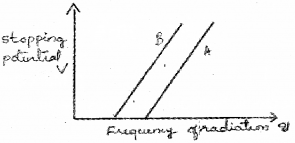
When photons incident on a metallic surface electric current is produced. The work function of a metal is given by ϕ0 = hc/λ0 = hu0. Where λ0, h, u0 have their usual meaning. (Say – 2014)
The graph given below shows the variation in stopping potential with frequency u of the incident radiation of the materials A and B.
a) Which material is more photo sensitive?
b) Which material has higher work function?
c) What happens to the slope of the graph when intensity of radiation increase? Why?
Answer:
a) The material having low work function is more sensitive to photo electric effect. In this case B has low work function. Hence B is more sensitive for photo electric effect.
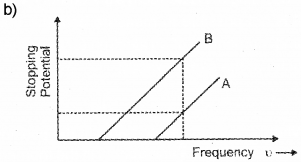
The above figure shows for a given frequency of radiation, B has more stoping potential than A. Which means that A has more work function than B.
c) The slope of this graph gives (h/c). h/e does not depend upon intensity. Hence slope of this graph does not change with intensity of incident light.
Question 10.
a) List out any two limitations of Bohr atom model. (March – 2016)
b) According to de-Broglie’s explanation of Bohr’s second postulate of quantization, the standing particle wave on a circular orbit for n = 4 is given by

Answer:
a) Limitations of Bohr atom model
i) The Bohr model is applicable to hydrogenic atoms. It cannot be extended to many electron atoms such as helium.
ii) The model is unable to explain the relative intensities of the frequencies in the spectrum.
iii) Bohr model could not explain fine structure of spectral lines.
b) 2πrn = 4λ, (iii)
Question 11.
The atomic hydrogen emits lines spectrum consisting of various series. (Say – 2016)
a) Name the series observed first.
b) Draw the energy level diagram of hydrogen atom.
Answer:
a) Layman series
b) Refer Qn. Ic, March 2012
Question 12.
a) Unit of wave raInberis (March – 2017)
(i) Hz
(ii) eV
(iii) m
(iv) m-1
b) Energy of ground state of hydrogen atom is -13.6 eV. What is its ionisation potential?
Answer:
a) iv) m-1
b) Ionisation energy is the minimum energy required to free the electron from the ground state of atom. The ionisation of energy of hydrogen atom = 13.8ev
Question 13.
The longest wavelength in Salmer series produced by atomic hydrogen is 656.4 nm. Find the shortest wavelength in this series. (Say – 2017)
Question 14.
a) Unitofwave number is (March – 2017)
(i) Hz
(ii) eV
(iii) m
(iv) rrr1
b) Energy of ground state of hydrogen atom is – 13.6 eV. What is its ionisation potential?
Answer:
a) iv) nr1
b) Ionisation energy is the minimum energy required to free the electron from the ground state of atom. The ionisation of energy of hydrogen atom = 13.6 ev
Question 15.
The longest wavelength in Balmer series produced by atomic hydrogen is 656.4 nm. Find the shortest wavelength in this series. (Say – 2017)
Answer:
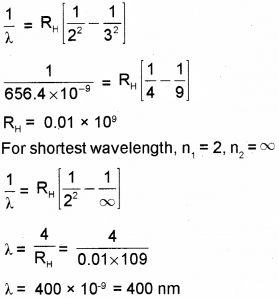
We hope the Kerala Plus Two Chemistry Chapter Wise Questions and Answers Chapter 12 Atoms help you. If you have any query regarding Kerala Plus Two Chemistry Chapter Wise Questions and Answers Chapter 12 Atoms, drop a comment below and we will get back to you at the earliest.