Why is an electrolyte able to conduct electricity while a Nonelectrolyte Cannot?
Electrolytes and Non-electrolytes
- Chemical substances can be classified into electrolytes and non-electrolytes.
- Electrolytes are substances that can conduct electricity either in the molten state or in an aqueous solution and undergo chemical changes.
- Non-electrolytes are substances that cannot conduct electricity either in the molten state or in an aqueous solution.
- Conductors are substances that can conduct electricity in the solid or molten state but are not chemically changed. Therefore, conductors are not electrolytes.
- Figure illustrates the particles in an electrolyte and a non-electrolyte.
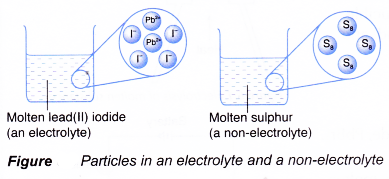
- In general,
(a) examples of electrolytes are acids, alkalis, salt solutions or molten salts. All these are ionic substances.
(b) examples of non-electrolytes are covalent substances which do not contain ions. - Ionic compounds do not conduct electricity in the solid state because the ions are held in a lattice and do not move freely. However, when they are melted or dissolved in water, they can conduct electricity. This is because the ions are free to move in the molten state or aqueous solution. For example, solid lead(II) iodide does not conduct electricity while molten lead(II) iodide does.
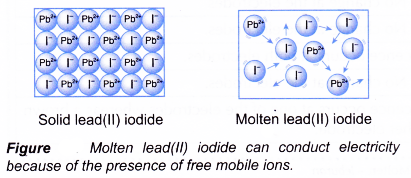
- Hydrogen chloride, HCl and ammonia, NH3 are covalent compounds. They exist as molecules in organic solvents. Hence, they do not conduct electricity in an organic solvent such as methylbenzene. However, solutions of hydrogen chloride and ammonia in water conduct electricity. This is because they exist as free mobile ions in water.
People also ask
- Analysing the electrolysis of molten compounds
- Analysing the Electrolysis of Aqueous Solutions
- What does electrochemical series mean?
- How does a voltaic cell work?
- How is electrolysis used in the industry?
Electrolytes and Non-electrolytes Experiment
Aim: To classify substances into electrolytes and non-electrolytes.
Materials: Solid lead(II) bromide, acetamide, naphthalene, sodium hydroxide solution, glucose solution and copper(II) sulphate solution.
Apparatus: Batteries, bulb, switch, connecting wires with crocodile clips, carbon electrodes with holders, crucible,
100 cm3 beaker, Bunsen burner, tripod stand and pipe-clay triangle.
Procedure:
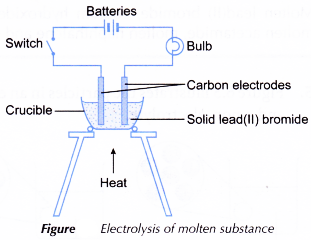
A. For molten substances
- Two-thirds of a crucible is filled with solid lead(II) bromide, PbBr2.
- The apparatus is set up as shown in Figure.
- The solid lead(II) bromide is heated until it is completely molten.
- The switch is turned on. The changes to the bulb and electrodes are observed.
- Steps 1 to 4 are repeated by replacing the solid lead(II) bromide with acetamide, CH3CONH2 and naphthalene, C10H8 respectively.
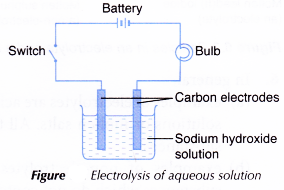
B. For aqueous solutions
- A beaker is filled with sodium hydroxide, NaOH solution until it is half full.
- The apparatus is set up as shown in Figure.
- The switch is turned on. The changes to the bulb and electrodes are observed.
- Steps 1 to 3 are repeated by replacing the sodium hydroxide solution with glucose, C6H12O6 solution and copper(II) sulphate, CuSO4 solution respectively.
Observations:
| Substance | Observation |
| Molten lead(ll) bromide | The bulb lights up. A brown gas is released at one of the electrodes. |
| Molten acetamide | The bulb does not light up. No change at the electrodes |
| Molten naphthalene | The bulb does not light up. No change at the electrodes. |
| Sodium hydroxide solution | The bulb lights up. Effervescence occurs at both electrodes. |
| Glucose solution | The bulb does not light up. No change at the electrodes. |
| Copper(ll) sulphate solution | The bulb lights up. Effervescence occurs at one of the electrodes whereas a brown solid is deposited on the other electrode. |
Discussion:
- Molten lead(II) bromide, sodium hydroxide solution and copper(II) sulphate solution can conduct electricity. This is because they contain free mobile ions which can carry electric current.
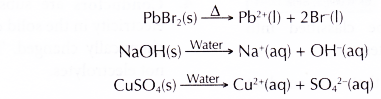
- The changes at the electrodes show that these substances are decomposed when electricity is passed through them.
- Molten acetamide, molten naphthalene and glucose solution cannot conduct electricity. This is because they do not contain ions, but are made up of molecules which cannot carry an electric current.
Conclusion:
Molten lead(II) bromide, sodium hydroxide solution and copper(II) sulphate solution are electrolytes whereas molten acetamide, molten naphthalene and glucose solution are non-electrolytes.