Analysing the Electrolysis of Aqueous Solutions
- An aqueous solution of a compound is a solution produced when the compound is dissolved in water.
- An aqueous solution of a compound contains
(a) anions and cations of the compound.
(b) hydrogen ions, H+ and hydroxide ions, OH– from the partial dissociation of water molecules.
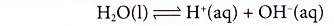
- During the electrolysis of an aqueous solution of a compound
(a) two different types of cation move towards the cathode, which are cations of the compound and hydrogen ions.
(b) two different types of anion move towards the anode, which are anions of the compound and hydroxide ions. - The electrolysis of an aqueous solution of sodium chloride is shown in Figure.
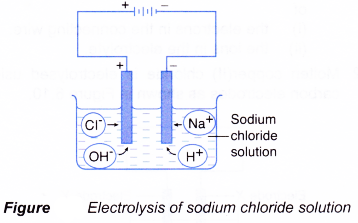
- The Na+ ions and H+ ions move towards the cathode.
- The Cl– ions and OH– ions move towards the anode.
- Only one type of ion will be selected to be discharged at the anode and cathode respectively.
- If H+ ions are selected to be discharged at the cathode, hydrogen gas will be released.
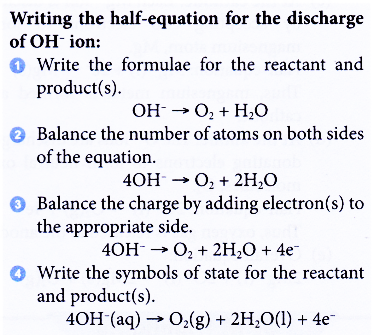
2H+(aq) + 2e– → H2(g) - If OH– ions are selected to be discharged at the anode, water and oxygen gas are produced.
4OH–(aq) → O2(g) + 2H2O(1) + 4e–
- The selective discharge of ions depends on three factors:
(a) positions of the ions in the electrochemical series
(b) concentration of the ions in the electrolyte
(c) types of electrodes used in the electrolysis - The electrochemical series is a list of ions arranged in ascending order of their tendency to discharge (Figure).
- The lower the position of an ion in the electrochemical series, the higher is the tendency of the ion to be discharged.
(i) Consider if the cations that move to the cathode are Cu2+ ions and H+ ions. The Cu2+ ion is lower than the H+ ion in the electrochemical series. Hence, the Cu2+ ions will be selectively discharged first.
(ii) Consider if the anions that move to the anode are OH– ions and SO42- ions. The OH– ion is lower than the SO42- ion in the electrochemical series. Hence, the OH– ions will be selectively discharged first.
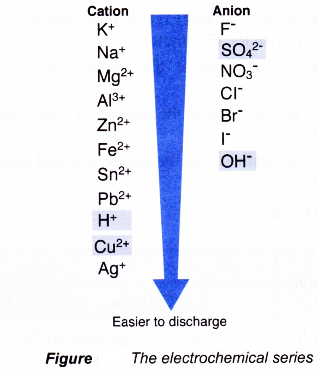
- The ions in the upper position of the electrochemical series are not selectively discharged to form atoms or molecules because these ions have a stronger tendency to exist as ions than atoms or molecules.
- The lower the position of an ion in the electrochemical series, the higher is the tendency of the ion to be discharged.
People also ask
- Why is an electrolyte able to conduct electricity while a Nonelectrolyte Cannot?
- Analysing the electrolysis of molten compounds
- What does electrochemical series mean?
- How does a voltaic cell work?
- How is electrolysis used in the industry?
Electrolysis of other concentrated aqueous solutions:
(a) Electrolysis of concentrated lead(II) nitrate solution
- Concentrated lead(II) nitrate, Pb(NO3)2 solution consists of Pb2+, H+, NO3– and OH– ions that move freely.
- The Pb2+ ions and H+ ions move to the cathode, while the NO3– ions and OH– ions move to the anode.
- At the cathode: The Pb2+ ions are selectively discharged because of their higher concentration in the electrolyte.
Pb2+(aq) + 2e– → Pb(s) - At the anode: The OH– ions are selectively discharged because their position in the electrochemical series is lower than the NO3– ions. Here, the concentration factor is unimportant.
4OH–(aq) → O2(g) + 2H2O(l) + 4e–
(b) Electrolysis of concentrated potassium iodide solution
- Concentrated potassium iodide, KI solution consists of K+, H+, I– and OH– ions that move freely.
- The K+ ions and H+ ions move to the cathode, while the I– ions and OH– ions move to the anode.
- At the cathode: The H+ ions are selectively discharged because their position in the electrochemical series is lower than the K+ ions. Here, the concentration factor is unimportant.
2H+(aq) + 2e– → H2(g) - At the anode: The I– ions are selectively discharged because of their higher concentration in the electrolyte.
2I–(aq) → I2(aq) + 2e–
Electrolysis of other aqueous solutions using active electrodes:
(a) Electrolysis of silver nitrate, AgNO3 solution using silver electrodes
- Silver nitrate solution consists of Ag+ ions, H+ ions, NO3 ions and OH– ions.
- At the cathode: The Ag+ ions and H+ ions move to the cathode. The Ag+ ion is lower than the H+ ion in the electrochemical series. Hence, the Ag+ ions are selectively discharged to form silver atoms. Silver metal is deposited on the cathode.
Ag+(aq) + e– → Ag(s) - At the anode: The NO– ions and OH– ions move to the anode. However, these ions are not discharged. Instead, the silver electrode dissolves to form Ag+ ions.
Ag(s) → Ag+(aq) + e– - Consequently, the concentration of the silver nitrate solution remains unchanged.
(b) Electrolysis of saturated sodium chloride, NaCl solution using graphite as the anode and mercury as the cathode
- Saturated sodium chloride solution consists of Na+ ions, H+ ions, Cl– ions and OH– ions.
- At the cathode: The Na+ ions and H+ ions move to the mercury cathode. The Na+ ions are selectively discharged to form sodium metal. The sodium formed then combines with mercury to form sodium amalgam.
Na+(aq) + e– → Na(l)
Na(l) + Hg(l) → Na/Hg(l) (Amalgam)
Even though Na+ ion is higher than H+ ion in the electrochemical series, the Na+ ions are selectively discharged because of the effect of the mercury electrode. - At the anode: The Cl– ions and OH– ions move to the graphite anode. The Cl– ions are selectively discharged because of their higher concentration in the electrolyte. Hence, chlorine gas is formed.
2Cl–(aq) → Cl2(g) + 2e–
Electrolysis of Copper(II) Sulphate Solution Experiment 1
Aim: To investigate the electrolysis of copper(II) sulphate solution and dilute sulphuric acid.
Materials: 0.1 mol dm-3 copper(II) sulphate solution, 0.1 mol dm-3 sulphuric acid and wooden splint.
Apparatus: Batteries, carbon electrodes, electrolytic cell, connecting wires with crocodile clips, ammeter, test tubes and switch.
Procedure:
A. Electrolysis of copper(II) sulphate solution
- An electrolytic cell is filled with 0.1 mol dm-3 copper(II) sulphate, CuSO4 solution until it is half full.
- The apparatus is set up as shown in Figure. The test tube must be full of the copper(II) sulphate solution at the beginning of the activity.
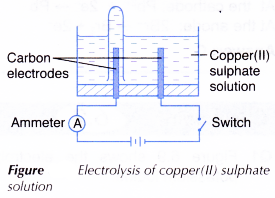
- The switch is turned on to allow electricity to pass through the electrolyte for 15 minutes.
- The observations at the anode, cathode and electrolyte are recorded.
- The gas gathered at the anode is tested using a glowing wooden splint.
B. Electrolysis of dilute sulphuric acid
- An electrolytic cell is filled with dilute sulphuric acid, H2SO4 until it is half full.
- The apparatus is set up as shown in Figure. The test tubes must be full of dilute sulphuric acid at the beginning of the activity.
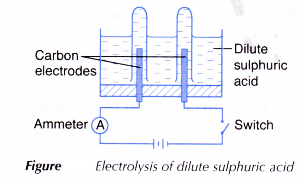
- The switch is turned on to allow electricity to pass through the electrolyte for 15 minutes.
- The observations at the anode, cathode and electrolyte are recorded.
- The gas gathered at the cathode is tested using a lighted wooden splint.
- The gas gathered at the anode is tested using a glowing wooden splint.
Observations:
| Electrolyte | Observation | ||
| Cathode | Anode | Change in solution | |
| Copper(II) sulphate solution | A brown solid is deposited on the cathode. | Gas bubbles are released. A colourless gas which relights a glowing wooden splint is produced. | The intensity of the blue colour of the electrolyte decreases. |
| Dilute sulphuric acid | Gas bubbles are released. A colourless gas is produced which gives a ‘pop’ sound when tested with a lighted wooden splint. | Gas bubbles are released. A colourless gas which relights a glowing wooden splint is produced. | The colourless solution remains unchanged. |
The ammeter needle is deflected.
Discussion:
- The aqueous solution of copper(II) sulphate consists of copper(II) ions, Cu2+, sulphate ions, SO42-, hydrogen ions, H+ and hydroxide ions, OH– that move freely.
- During the electrolysis, the Cu2+ ions and H+ ions move to the cathode. The Cu2+ ions are selectively discharged whereby each Cu2+ ion accepts two electrons to form copper metal.
Cu2+(aq) + 2e– → Cu(s) - The SO42- ions and OH– ions move to the anode. The OH– ions are selectively discharged by donating electrons to form oxygen and water.
4OH–(aq) → O2(g) + 2H2O(I) + 4e– - The intensity of the blue colour of the electrolyte decreases as the concentration of blue Cu2+ ions decreases when more copper is deposited on the cathode.
- The electrolyte becomes more acidic because of the H+ ions and SO42- ions left.
- During the electrolysis, the Cu2+ ions and H+ ions move to the cathode. The Cu2+ ions are selectively discharged whereby each Cu2+ ion accepts two electrons to form copper metal.
- Dilute sulphuric acid consists of hydrogen ions, H+, sulphate ions, SO42- and hydroxide ions, OH– that move freely.
- During the electrolysis, the H+ ions move to the cathode. The H+ ions are discharged by accepting electrons to form hydrogen gas.
2H+(aq) + 2e– → H2(g) - The S042- ions and OH– ions move to the anode. The OH– ions are selectively discharged by donating electrons to form oxygen and water.
4OH–(aq) → O2(g) + 2H2O(I) + 4e– - The concentration of sulphuric acid increases gradually as water is decomposed to hydrogen gas and oxygen gas. The volume of hydrogen gas formed is twice the volume of oxygen gas.
- During the electrolysis, the H+ ions move to the cathode. The H+ ions are discharged by accepting electrons to form hydrogen gas.
Conclusion:
- During the electrolysis of copper(II) sulphate solution using carbon electrodes, copper metal is deposited at the cathode and oxygen gas is produced at the anode.
- During the electrolysis of dilute sulphuric acid using carbon electrodes, hydrogen gas is given off at the cathode and oxygen gas is produced at the anode.
Electrolysis of Copper(II) Sulphate Solution Experiment 2
Aim: To investigate the effect of the types of electrodes on the products of electrolysis.
Problem statement: Do the types of electrodes affect the types of products formed during the electrolysis?
Hypothesis: When copper electrodes are used instead of carbon electrodes during the electrolysis of copper(II) sulphate solution, the types of products formed at the electrodes are different.
Variables:
(a) Manipulated variable : Types of electrodes
(b) Responding variable : Products formed at the electrodes
(c) Controlled variables : Type and concentration of the electrolyte
Materials: 0.1 mol dm-3 copper(II) sulphate solution, wooden splint and sandpaper.
Apparatus: Batteries, carbon electrodes, copper electrodes, electrolytic cell, connecting wires with crocodile clips, ammeter, test tube and switch.
Procedure:
- Two carbon electrodes are cleaned with sandpaper.
- An electrolytic cell is filled with 0.1 mol dm-3 copper(II) sulphate, CuSO4 solution until it is half full.
- The apparatus is set up as shown in Figure.
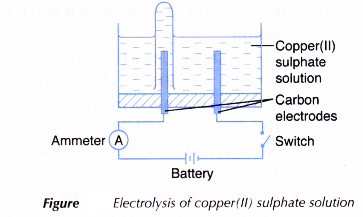
- The switch is turned on to allow electricity to pass through the electrolyte for 15 minutes.
- The observations at the anode, cathode and electrolyte are recorded.
- Steps 1 to 5 are repeated using copper electrodes to replace the carbon electrodes.
Observations:
| Electrode | Observation | ||
| Anode | Cathode | Electrolyte | |
| Carbon | Gas bubbles are released. A colourless gas which relights a glowing wooden splint is produced. | A brown solid is deposited on the cathode. | The intensity of the blue colour of the electrolyte decreases. |
| Copper | The copper electrode dissolves into the solution. The anode becomes thinner. | The cathode becomes thicker. | The intensity of the blue colour of the electrolyte remains unchanged. |
Discussion:
- The aqueous solution of copper(II) sulphate consists of copper(II) ions, Cu2+, sulphate ions, SO42-, hydrogen ions, H+ and hydroxide ions, OH– that move freely.
- During the electrolysis using carbon electrodes,
- The Cu2+ ions and H+ ions move to the cathode. The Cu2+ ion is lower than the H+ ion in the electrochemical series. Hence, the Cu2+ ions are selectively discharged to form copper metal.
Cu2+(aq) + 2e– Cu(s) - The SO42- ions and OH– ions move to the anode. The OH– ion is lower than the SO42- ion in the electrochemical series. Hence, the OH– ions are selectively discharged to form oxygen and water.
4OH–(aq) → O2(g) + 2H2O(I) + 4e– - The intensity of the blue colour of the electrolyte decreases because the concentration of the blue Cu2+ ions decreases when more copper is deposited on the cathode.
- The Cu2+ ions and H+ ions move to the cathode. The Cu2+ ion is lower than the H+ ion in the electrochemical series. Hence, the Cu2+ ions are selectively discharged to form copper metal.
- During the electrolysis using copper electrodes,
- The Cu2+ ions and H+ ions move to the cathode. The Cu2+ ion is lower than the H+ ion in the electrochemical series. Hence, the Cu2+ ions are selectively discharged to form copper metal.
Cu2+(aq) + 2e– → Cu(s) - The SO42- ions and OH– ions move to the anode. However, these ions are not discharged. Instead, the copper electrode dissolves to form copper(II) ions.
Cu(s) → Cu2+(aq) + 2e– - The intensity of the blue colour of the electrolyte remains unchanged. This is because the concentration of the blue Cu2+ ions remains unchanged. For one Cu2+ ion discharged to form a copper atom at the cathode, one copper atom from the anode will dissolve to form a Cu2+ ion.
- The Cu2+ ions and H+ ions move to the cathode. The Cu2+ ion is lower than the H+ ion in the electrochemical series. Hence, the Cu2+ ions are selectively discharged to form copper metal.
Conclusion:
During the electrolysis of copper(II) sulphate solution, oxygen and water are formed at the anode when carbon electrodes are used, while the copper anode dissolves to form copper(II) ions when copper electrodes are used. However, the product formed at the cathodes is not affected by the type of electrodes used. The hypothesis is accepted.
Electrolysis of Silver Nitrate Solution Experiment
Aim: To investigate the effect of the positions of ions in the electrochemical series on the products of electrolysis.
Problem statement: How do the positions of ions in the electrochemical series affect the selective discharge of ions at the electrodes?
Hypothesis: The lower the position of an ion in the electrochemical series, the higher is the tendency of that ion to be discharged.
Variables:
(a) Manipulated variable : Positions of ions in the electrochemical series
(b) Responding variable : Ions discharged at the electrodes
(c) Controlled variables : Concentration of electrolyte, types of electrodes, duration of electrolysis
Materials: 0.1 mol dm-3 silver nitrate solution, 0.1 mol dm-3 sodium sulphate solution and wooden splint.
Apparatus: Batteries, carbon electrodes, electrolytic cell, connecting wires with crocodile clips, ammeter, test tubes and switch.
Procedure:
- An electrolytic cell is filled with 0.1 mol dm-3 silver nitrate, AgNO3 solution until it is half full.
- The apparatus is set up as shown in Figure.
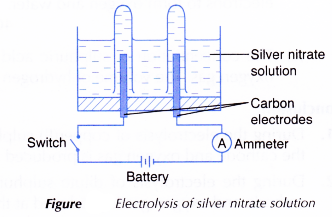
- The switch is turned on to allow electricity to pass through the electrolyte for 15 minutes.
- The observations at the anode and cathode are recorded.
- Any gases produced are tested using a wooden splinter.
- Steps 1 to 5 are repeated using 0.1 mol dm-3 sodium sulphate, Na2SO4 solution to replace 0.1 mol dm-3 silver nitrate solution.
Observations:
| Electrolyte | Observation | |
| Cathode | Anode | |
| Silver nitrate solution | A shiny grey solid is deposited on the cathode. | Gas bubbles are released. A colourless gas which relights a glowing wooden splint is produced. |
| Sodium sulphate solution | Gas bubbles are released. A colourless gas is produced which gives a ‘pop’ sound when tested with a lighted wooden splint. | Gas bubbles are released. A colourless gas which relights a glowing wooden splint is produced. |
Discussion:
- The aqueous solution of silver nitrate consists of silver ions, Ag+, nitrate ions, NO3–, hydrogen ions, H+ and
hydroxide ions, OH– that move freely.- During the electrolysis, the Ag+ ions and H+ ions move to the cathode. The Ag+ ion is lower than the H+ ion in the electrochemical series. Hence, the Ag+ ions are selectively discharged by accepting electrons to form silver atoms.
Ag+(aq) + e– → Ag(s) - The NO3– ions and OH– ions move to the anode. The OH– ion is lower than the NO3– ion in the electrochemical series. Hence, OH– ions are selectively discharged by donating electrons to form oxygen and water.
4OH–(aq) → O2(g) + 2H2O(l) + 4e– - Consequently, the electrolyte gradually becomes more acidic because of the H+ ions and NO3– ions left.
- During the electrolysis, the Ag+ ions and H+ ions move to the cathode. The Ag+ ion is lower than the H+ ion in the electrochemical series. Hence, the Ag+ ions are selectively discharged by accepting electrons to form silver atoms.
- The aqueous solution of sodium sulphate consists of sodium ions, Na+, sulphate ions, SO42-, hydrogen ions, H+ and hydroxide ions, OH– that move freely.
- During the electrolysis, the Na+ ions and H+ ions move to the cathode. The H+ ion is lower than the Na+ ion in the electrochemical series. Hence, the H+ ions are selectively discharged by accepting electrons to form hydrogen gas.
2H+(aq) + 2e– → H2(g) - The SO42- ions and OH– ions move to the anode. The OH– ion is lower than the SO42- ion in the electrochemical series. Hence, the OH- ions are selectively discharged by donating electrons to form oxygen and water.
4OH–(aq) → O2(g) + 2H2O(l) + 4e– - The concentration of sodium sulphate solution increases gradually as water is decomposed to hydrogen gas and oxygen gas.
- During the electrolysis, the Na+ ions and H+ ions move to the cathode. The H+ ion is lower than the Na+ ion in the electrochemical series. Hence, the H+ ions are selectively discharged by accepting electrons to form hydrogen gas.
Conclusion:
The ions at the lower position of the electrochemical series will be selectively discharged during the electrolysis of silver nitrate solution and sodium sulphate solution. The hypothesis is accepted.
Electrolysis of Hydrochloric Acid Solution Experiment
Aim: To investigate the effect of the concentration of ions in a solution on the products of electrolysis.
Problem statement: How does the concentration of ions in hydrochloric acid affect the discharge of ions at the anode?
Hypothesis: When the concentration of chloride ions is higher, chloride ions will be selectively discharged at the anode during the electrolysis of hydrochloric acid.
Variables:
(a) Manipulated variable : Concentration of ions in the solution
(b) Responding variable : Types of ions to be discharged at the electrode
(c) Controlled variables : Type of electrolyte, types of electrodes, duration of electrolysis 0.001 mol dm-3 hydrochloric acid, 2 mol dm-3 hydrochloric acid, litmus paper and wooden splint.
Apparatus: Batteries, carbon electrodes, electrolytic cell, connecting wires with crocodile clips, ammeter, test tubes and switch.
Procedure:
- An electrolytic cell is filled with 0.001 mol dm-3 hydrochloric acid, HCl until it is half full.
- The apparatus is set up as shown in Figure.
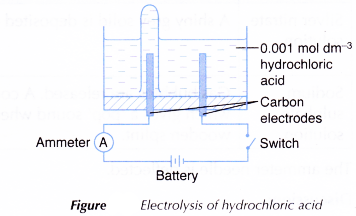
- The switch is turned on to allow electricity to pass through the electrolyte for 15 minutes.
- The observation at the anode is recorded.
- Any gas produced at the anode is tested.
- Steps 1 to 5 are repeated using 2 mol dm-3 hydrochloric acid to replace 0.001 mol dm-3 hydrochloric acid.
Observations:
| Solution | Observation at the anode |
| 0.001 mol dm-3 hydrochloric acid | Gas bubbles are released. A colourless gas which relights a glowing wooden splint is produced. |
| 2 mol dm-3 hydrochloric acid | A greenish-yellow gas with a pungent and choking smell is released. The gas turns the blue litmus paper red and then white. |
Discussion:
- The aqueous solution of hydrochloric acid consists of hydrogen ions, H+, chloride ions, Cl– and hydroxide ions, OH– that move freely.
- During the electrolysis, the Cl– ions and OH– ions move to the anode.
- Electrolysis of 0.001 mol dm-3 hydrochloric acid:
The OH– ions are selectively discharged to form oxygen and water. This is because the OH– ion is lower than the Cl– ion in the electrochemical series.
4OH–(aq) → O2(g) + 2H2O(I) + 4e– - Electrolysis of 2 mol dm-3 hydrochloric acid:
The Cl– ions are selectively discharged because of their higher concentration in the electrolyte even though the Cl– ion is higher than the OH– ion in the electrochemical series. Hence, chlorine gas is formed.
2Cl–(aq) → Cl2(g) + 2e–
- Electrolysis of 0.001 mol dm-3 hydrochloric acid:
Note:
During the electrolysis, only the H+ ions move to the cathode. Hence, the H+ ions are discharged to form hydrogen gas.
2H+(aq) + 2e– → H2(g)
During the electrolysis of 0.001 mol dm-3 hydrochloric acid, the concentration of the electrolyte increases gradually as water is decomposed to hydrogen gas and oxygen gas.
During the electrolysis of 2 mol dm-3 hydrochloric acid, the concentration of the electrolyte decreases gradually as H+ ions and Cl– ions are removed from the electrolyte.
Conclusion:
During the electrolysis of 2 mol dm-3 hydrochloric acid, the selective discharge of chloride ions is affected by the concentration of the ions instead of its position in the electrochemical series. The hypothesis is accepted.