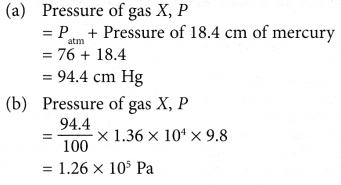Understanding Gas Pressure
- Figure shows a boy pressing the front tyre of his bicycle.
 (a) When he presses the tyre with his thumb, he feels an opposing force acting on his thumb.
(a) When he presses the tyre with his thumb, he feels an opposing force acting on his thumb.
(b) The force is due to the air pressure in the tyre.
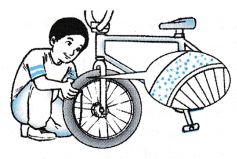
(c) The air pressure is due to the collisions of air molecules in the tyre against the wall of the tyre. - Figure shows air molecules in an enclosed container. Air pressure can be explained by the kinetic theory.
 (a) When an air molecule hits the wall of a container, it bounces back.
(a) When an air molecule hits the wall of a container, it bounces back.
(b) The change of momentum per second gives the force exerted on the wall by the molecule.
(c) With a large number of molecules hitting the container wall, a large force is exerted on it.
(d) By using the formula for pressure, P = F/A, the force per unit area gives the pressure exerted by the air on the wall.
Instruments for Measuring Gas Pressure
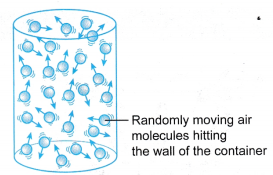
- Figure shows a U-tube manometer which can be used to measure gas pressure.
 (a) The gas whose pressure is to be measured is connected directly to one side of the U-tube.
(a) The gas whose pressure is to be measured is connected directly to one side of the U-tube.
(b) The pressure of the gas, P is equal to the sum of atmospheric pressure and the pressure due to the difference in the height, h of the liquid in the manometer.
(c) The pressure of the gas P:
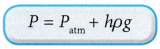 Where ρ is the density of the liquid in the manometer.
Where ρ is the density of the liquid in the manometer. - Figure shows a Bourdon gauge commonly used in school laboratories, air conditioner service shops, hospitals and factories.
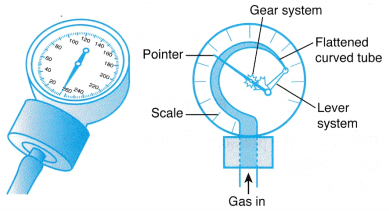 (a) When a Bourdon gauge is attached to a gas cylinder, gas flows from the cylinder into the flattened curved tube in the gauge.
(a) When a Bourdon gauge is attached to a gas cylinder, gas flows from the cylinder into the flattened curved tube in the gauge.
(b) The pressure of the gas causes the curved tube to straighten out.
(c) Through a lever and a gear system, the pointer rotates to measure the pressure of the gas. The scale may be calibrated in N m-2 or Pa.
Example 1. Figure shows a U-tube manometer used to measure the pressure of gas X.
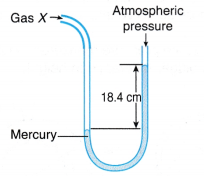
Determine the pressure of gas X in
(a) cm Hg,
(b) pascal.
[Density of mercury, ρ = 1.36 x 104 kg m-3; g = 9.8 N kg-1; Patm= 76 cm Hg]
Solution: