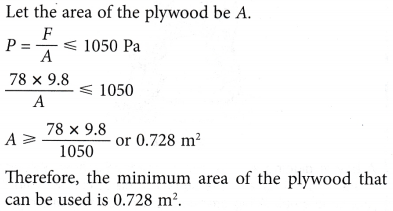
Understanding Pressure Using Pascal’s Principle
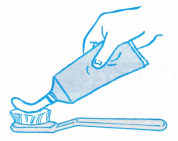
- A boy applies pressure on a tube of toothpaste. The pressure applied to the tube is transmitted throughout the toothpaste and forces it out of the opening of the tube.
- Figure shows a boy holding a plastic bag with holes. When he squeezes the plastic bag, the water squirts from all the holes in all directions.
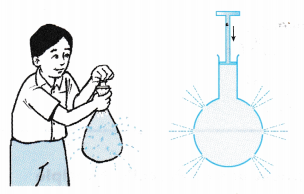
- Figure shows an apparatus that consists of a glass barrel fitted to a plunger and ending with a bulb with holes of uniform size. This apparatus is fully filled with water. When the plunger is pushed in, water squirts equally from all the holes.
- From above Figures, it is shown that
(a) when pressure is applied on a liquid, it is transmitted throughout the liquid.
(b) the pressure acts in all directions. - Pascal’s principle states that pressure applied to an enclosed fluid is transmitted uniformly to every part of the fluid and to the walls of the container of the fluid.
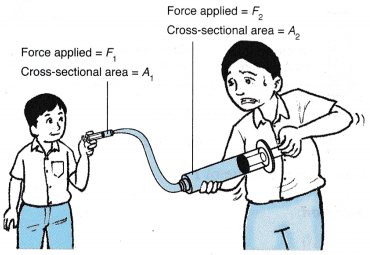
- Figure shows a big and a small syringe connected by a rubber tube. The syringes and rubber tube are filled with water.
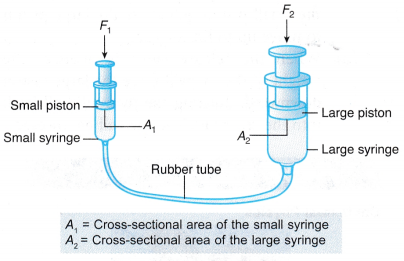 (a) A force, F1 acting on the small syringe is balanced by a force, F2 acting on the large syringe.
(a) A force, F1 acting on the small syringe is balanced by a force, F2 acting on the large syringe.
(b) According to Pascal’s principle, the pressure Fj, exerted by the small piston, on the water is equal to the pressure, P, exerted by the large piston on the water.
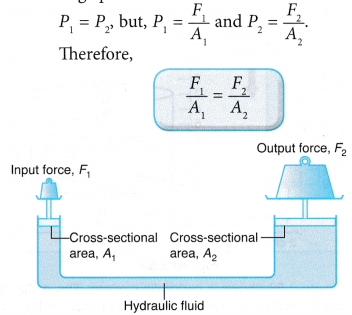
- Pascal’s principle can be used as a force multiplier in a hydraulic system. Figure shows a small input force, F1 on the small piston can result in a big output force, F2 on the big piston. From the above formula, we get:

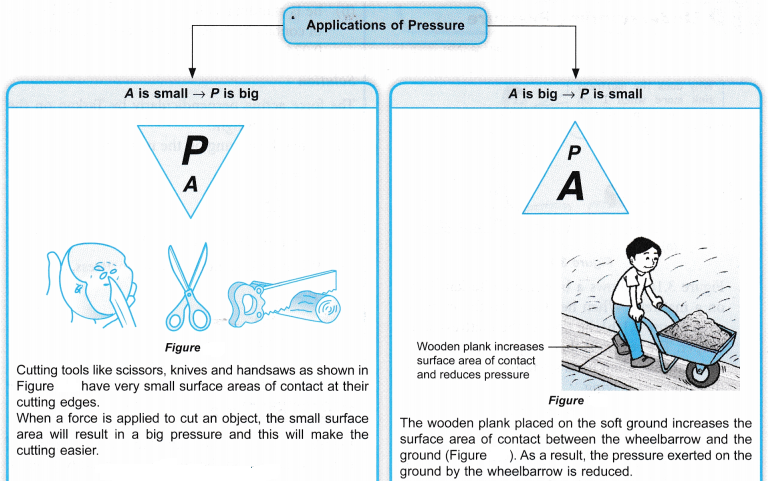
Applications of Pascal’s Principle
- Figure shows a hydraulic jack lifting the back portion of a car.
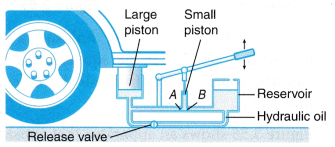 (a) When the small piston is pulled up, valve B opens and valve A closes. Hydraulic oil is drawn from the reservoir into the space under the small piston.
(a) When the small piston is pulled up, valve B opens and valve A closes. Hydraulic oil is drawn from the reservoir into the space under the small piston.
(b) When the small piston is pushed down, valve A opens and valve B closes. Hydraulic oil is forced through valve A and raises the large piston.
(c) Continuous up-and-down movements of the small piston will cause the large piston to move up to lift the car.
(d) When the release valve is opened, the weight of the car will force the large piston downward, pushing the hydraulic oil back into the reservoir. The car will be lowered. - An automobile hydraulic brake system consists of a master cylinder attached to the brake pedal and various slaves cylinders near the wheels.
Figure shows the brake system of a car.
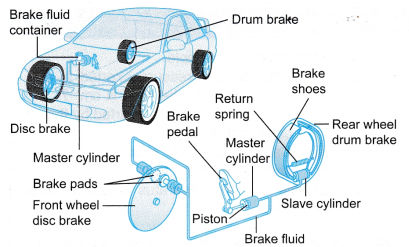 (a) When the brake pedal of a car is pressed, pressure is exerted on the brake fluid (oil) and is transmitted uniformly from the master cylinder, through the pipe, to the slave cylinders near the wheels.
(a) When the brake pedal of a car is pressed, pressure is exerted on the brake fluid (oil) and is transmitted uniformly from the master cylinder, through the pipe, to the slave cylinders near the wheels.
(b) This will move the slave pistons forward, forcing the brake pads against the discs or drums attached to the wheels to slow down the car.
(c) However, it is important to note that this system works only because of the fact that the brake fluid is incompressible.
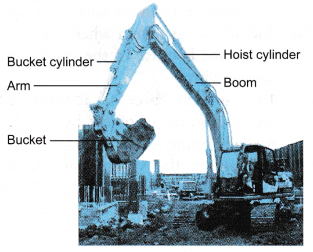
(d) If there is an air bubble in the system, then the pressure applied to the piston in the master cylinder will not be effectively transferred to the slave cylinders as the air bubble is compressible. This will affect the moving of the pistons in the slave cylinders and result in the car not being able to stop or slow down effectively. - A power shovel applies the Pascal’s principle in its operation. Figure shows a power shovel at work. It uses hydraulic cylinders to manipulate its boom and bucket to dig, lift or dump material.
Pascal’s Principle Example Problems with Solutions
Example 1. In the hydraulic system shown in Figure, an input force of 10 N is used to balance an output force, F2.
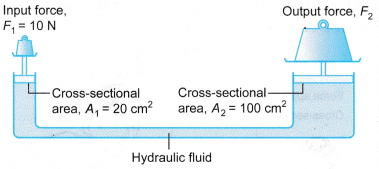
Calculate the value of F2.
Solution:

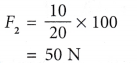
Example 2. Figure shows an activity to demonstrate the hydraulic system as a force multiplier. A small boy uses a force of 8 N to press the piston of the small syringe with a cross-sectional area of 1.6 cm2. His elder brother presses the piston of the large syringe with a cross-sectional area of 64 cm2.
What is the minimum force that the. elder brother needs to apply so that the piston of the large syringe will not be forced to move backward?
Solution:
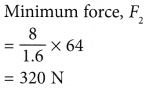
 (a) When he presses the tyre with his thumb, he feels an opposing force acting on his thumb.
(a) When he presses the tyre with his thumb, he feels an opposing force acting on his thumb.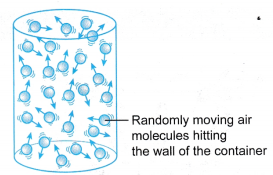 (a) When an air molecule hits the wall of a container, it bounces back.
(a) When an air molecule hits the wall of a container, it bounces back.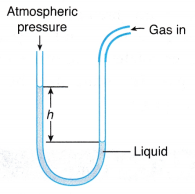 (a) The gas whose pressure is to be measured is connected directly to one side of the U-tube.
(a) The gas whose pressure is to be measured is connected directly to one side of the U-tube.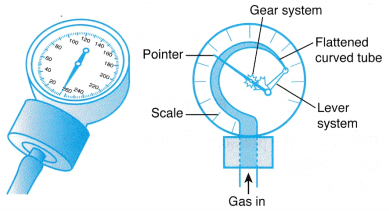 (a) When a Bourdon gauge is attached to a gas cylinder, gas flows from the cylinder into the flattened curved tube in the gauge.
(a) When a Bourdon gauge is attached to a gas cylinder, gas flows from the cylinder into the flattened curved tube in the gauge.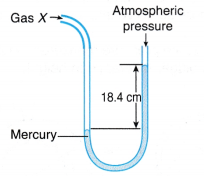




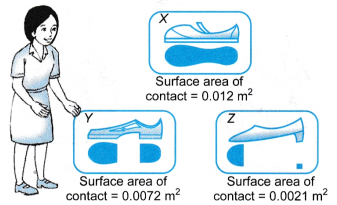


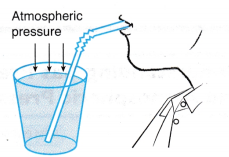 (a) He sucks the air in the straw and creates a partial vacuum in it.
(a) He sucks the air in the straw and creates a partial vacuum in it.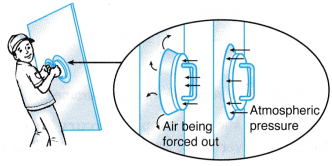 (a) When he presses the suction cups against the glass, air is forced out of the cups creating a partial vacuum in it.
(a) When he presses the suction cups against the glass, air is forced out of the cups creating a partial vacuum in it.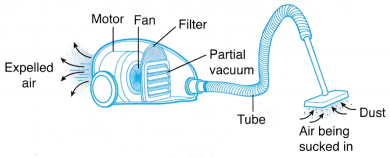 (a) The rotating fan expels air, creating a partial vacuum in the space in front of it.
(a) The rotating fan expels air, creating a partial vacuum in the space in front of it.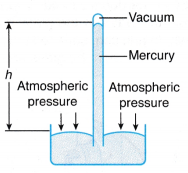 (a) Due to the inversion, a vacuum is created at the base of the tube and a column of mercury is supported by the atmospheric pressure.
(a) Due to the inversion, a vacuum is created at the base of the tube and a column of mercury is supported by the atmospheric pressure. where, ρ is the density of mercury.
where, ρ is the density of mercury.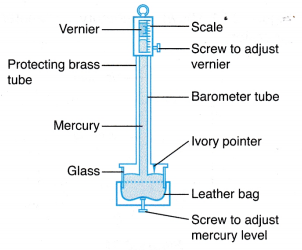 (a) The Fortin barometer has a metal scale with a vernier attached and a mirror behind the top of the mercury column to avoid parallax error when taking readings.
(a) The Fortin barometer has a metal scale with a vernier attached and a mirror behind the top of the mercury column to avoid parallax error when taking readings.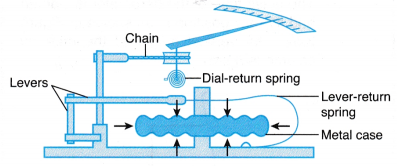 (a) Inside an aneroid barometer there is a partially evacuated metal case made of thin and flexible alloy.
(a) Inside an aneroid barometer there is a partially evacuated metal case made of thin and flexible alloy.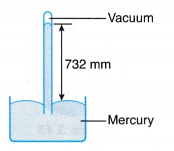

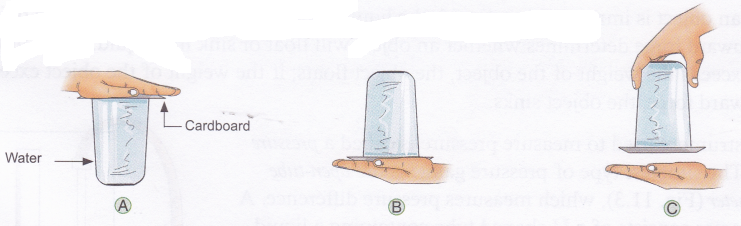
 Observation: You will see that it is really difficult to pull the rubber sucker off the smooth surface.
Observation: You will see that it is really difficult to pull the rubber sucker off the smooth surface.