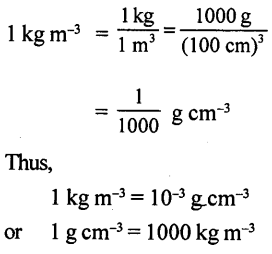
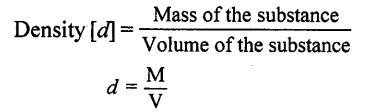Selina Concise Physics Class 8 ICSE Solutions – Matter
ICSE SolutionsSelina ICSE SolutionsML Aggarwal Solutions
APIusTopper.com provides step by step solutions for Selina Concise ICSE Solutions for Class 8 Physics Chapter 1 Matter. You can download the Selina Concise Physics ICSE Solutions for Class 8 with Free PDF download option. Selina Publishers Concise Physics for Class 8 ICSE Solutions all questions are solved and explained by expert teachers as per ICSE board guidelines.
Selina Class 8 Physics ICSE SolutionsChemistryBiologyMathsGeographyHistory & Civics
Selina Concise ICSE Solutions for Class 8 Physics Chapter 1 Matter
- Matter Every substance living and non-living that we see is made up of matter and MATTER “is something which has mass, occupies space and can be perceived by our senses.” e.g. Hydrogen, milk, oxygen, pen, table, water, iron, air, oil, sugar etc.
- Matter is composed of tiny particles called molecules, which are in constant motion, have spaces between them and have inter-molecular attraction.
- Every molecule can exist freely in nature and has all the properties of matter.
- A molecule is composed of ATOMS, but atom cannot exist free in nature.
- INTER-MOLECULAR FORCE ‘The molecules of a matter exert a force of attraction on each other – The force of attraction is called INTER-
- MOLECULAR FORCE This force in solid is very strong and we cannot break a solid easily. In liquids this force is less strong and in molecules of gas it is very less. –
- FORCE OF COHESION “The inter-molecular force of attraction between the molecules of same substance is called FORCE OF COHESION.” i.e. between water and water.
- FORCE OF ADHESION “The force of attraction between the molecules of two different substances is called FORCE OF ADHESION” i.e. between glue and paper.
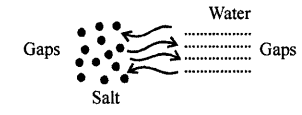
Matter is composed of tiny particles and molecules of matter have spaces between them can be proved by experiment.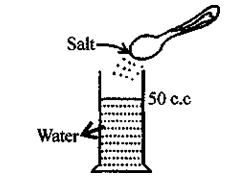
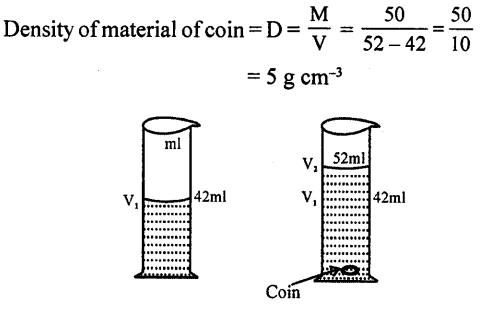
Take 50 c.c of water in a measuring cylinder. Add a small quantity of salt in it. Salt gets dissolved in water and still level remains at 50 c.c. Where has salt gone?
The salt molecules enter into spaces of water and water molecules into spaces of salt molecules. This experiment show that particles of matter are very minute and cannot be seen by naked eye and there are spaces between molecules.
- The molecules of matter are in constant motion can be seen by opening a bottle of perfume in a comer of room, it reaches the other parts of the room.
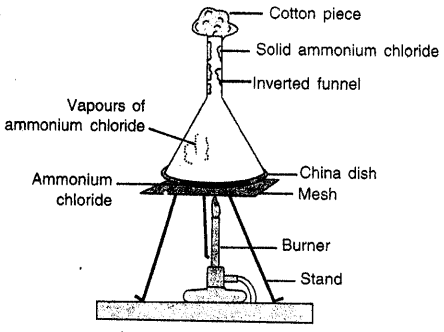
- SUBLIMATION Change of solid directly into vapours on absorbing heat.
- DEPOSITION “The change of vapours directly into solid on cooling.”
- MELTING “Change of solid in liquid at fixed temperature on heating.”
- FUSION or FREEZING “Change of liquid to solid state on cooling at a fixed temperature.”
- FUSION or MELTING “Change of a solid to liquid state at a fixed temperature on absorbing heat.”
- EVAPORATION Surface phenomenon i.e. only takes place at surface “Is change of liquid to vapours.” Evaporation has cooling effect. Takes place at all temperatures.
- VAPORIZATION “Change of liquid to vapour state on heating at constant temperature.”
It is fast process and produces hotness.
Test Yourself
A. Objective Questions
1. Write true or false for each statement
(a) The temperature of a substance remains unaffected during its change of state.
Answer: True.
(b) Ice melts at 100°C.
Answer: False. The ice melts at 0° by absorption of heat.
(c) Water at 100°C has more heat than the steam at 100°C.
Answer: False.
(d) Evaporation of a liquid causes cooling.
Answer: True.
(e) Water evaporates only at 100°C.
Answer: False.
(f) Boiling takes place at all temperatures.
Answer: False.
(g) Evaporation takes place over the entire mass of the liquid.
Answer: False.
(h) The process of a gas converting directly into solid is called vaporization.
Answer: False.
The process of a liquid converting directly into gas is called vaporization.
(i) At high altitudes water boils above 100° C.
Answer: False.
(j) The melting point of ice is 0°C.
Answer: True.
2. Fill in the blanks
(a) Evaporation takes place at all temperature.
(b) Freezing process is just reverse of melting.
(c) Sublimation is a process that involves direct conversion of a solid into its vapour on heating.
(d) The temperature at which a solid converts into a liquid is called its melting point.
(e) The smallest unit of matter that exists freely in nature is called molecule.
(f) Molecules of a substance are always in a state of motion and so they possess kinetic energy.
(g) Intermolecular space is maximum in gases less in liquids and the least in solids.
(h) Intermolecular force of attraction is maxiumum in solids, less in liquids and the least in gases.
3. Match the following
4. Select the correct alternative
(a) The inter-molecular force is maximum in
- solids
- gases
- liquids
- none of the above
(b) The inter-molecular space is maximum in
- liquids
- solids
- gases
- none of the above
(c) The molecules can move freely anywhere in
- gases
- liquids
- solids
- none of the above
(d) The molecules move only within the boundary of
- liquids
- gases
- solids
- none of the above
(e) The temperature at which a liquid gets converted into its vapour state is called its
- melting point
- boiling point
- dewpoint
- freezing point.
(f) Rapid conversion of water into steam is an example of
- evaporation
- freezing
- melting
- vapourization.
(g) Evaporation takes place from the
- surface of liquid
- throughout the liquid
- mid-portion of the liquid
- bottom of liquid.
(h) Boiling takes place from the
- the surface of the liquid
- throughout the liquid
- mid-portion of liquid
- none of the above.
Short/Long Answer Questions
Question 1.
Define the term matter. What is it composed of ?
Answer:
Anything which occupies space and has mass is called matter. Matter is composed of tiny particles called MOLECULES.
Question 2.
State three properties of molecules of a matter.
Answer:
- They are very small in size.
- They have spaces between them.
- They are in constant motion and they posses kinetic energy.
Question 3.
What do you mean by the inter-molecular spaces ? How do they vary in different states of matter ?
Answer:
INTER-MOLECULAR SPACES “The spacing between the molecules of matter is called Inter-molecular spaces.”
The inter-molecular spaces is less in solids more in liquids and still more in gases.
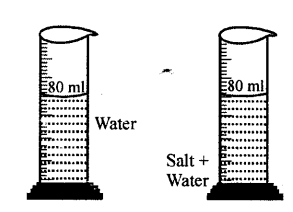
Explanation of inter-molecular space : Take water in a measuring cylinder say upto 80 ml. mark. Add 10 gm of salt to it. The volume in cylinder should increase. On dissolving salt we find volume remains same i.e. upto 80 ml mark. This is because there are spaces in water molecules and salt molecules occupy these spaces and volume remains the same.
Question 4.
What is meant by the inter-molecular forces of attraction ?
Answer:
How do they vary in solids, liquids and gases ?
INTER-MOLECULAR FORCES OF ATTRACTION : “The forces of attraction between the molecules of matter is called the inter-molecular force of attraction.”
This inter molecular force is maximum in solids, less in liquids and least in gases.
Question 5.
Which of the following are correct ?
Answer:
(a) Solids have definite shape and definite volume.
True.
Reason As the molecules here have negligible intermolecular distance between them and have maximum intermolecular force of attraction.
(b) Liquids have definite volume but do not have definite shape.
True.
(c) Gases have definite volume but no definite shape.
False.
Correct Gases have neither definite volume nor a definite shape.
(d) Liquids have definite shape and definite volume.
False.
Correct Liquids have a definite volume but not definite shape.
Question 6.
Discuss the three states of matter solid, liquids and gas on the basis of molecular model.
Answer:
Solids
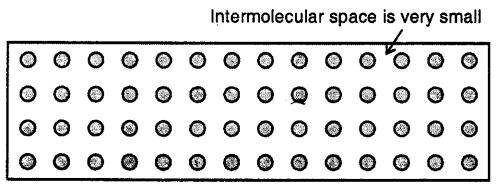
Here the molecules are very tightly packed that there is no or very less intermolecular space and there is high intermolecular force of attraction (force of cohesion). The molecules do not move about their mean position and thus solids have a definite shape and volume.
Liquids :
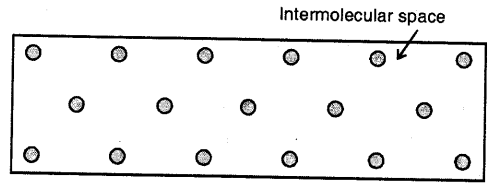
Here the molecules are less tightly packed as compared to solids and also there is lesser force of intermolecular attraction. The intermolecular distance is greater than that in the solids. Thus, they do not have a definite shape but acquire the shape of the vessel in which they are contained but have a definite volume at a given temperature.
Gases :
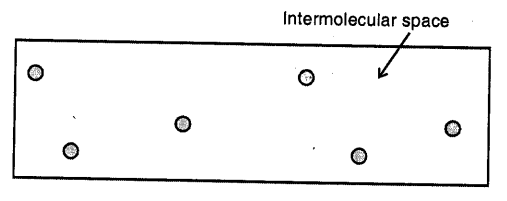
Here the molecules are far apart from each other i.e. have the greatest intermolecular distance which result into the weakest intermolecular forces of attraction. The molecules as are not bound by any strong force, move about freely and thus gases do not have a definite shape and’hlso do not have any definite volume.
Question 7.
What do you mean by the change of state ? Write the flow chart showing the complete cycle of change of state.
Answer:
CHANGE OF STATE: “The process of change from one state(form) to another state (form) either by absorption or rejection of heat at a constant temperature is called the CHANGE OF STATE.”
COMPLETE CYCLE OF CHANGE OF STATE : On heating a solid changes to liquid and liquid on heating changes to vapours. On cooling vapours condense to LIQUID, LIQUIDS on cooling freeze to SOLIDS. Some SOLIDS on heating change to vapours. On rejection of heat vapours solidify.
This cycle can be shown by diagram
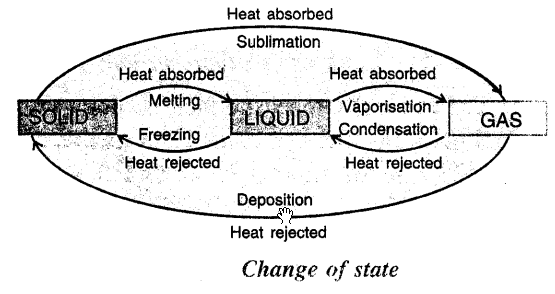
Question 8.
Differentiate between melting point and boiling point, giving atleast one example of each.
Answer:
MELTING POINT:
The temperature at which a solid starts changing into LIQUID without further increase in temperature is called MELTING POINT.” Or The constant temperature at which a solid changes into liquid.”
Example : Ice (solid) melts at Q?C into water (liquid) when heated.
BOILING POINT : “The temperature at which a LIQUID start changing in vapour without further rise in temperature.
Or
‘The constant temperature at which a LIQUID starts changing into GAS (vapours)
Example : Boiling point of water (liquid) is 100°C.
Question 9.
Describe the process of condensation and sublimation with examples.
Answer:
CONDENSATION :
“The change of vapours on cooling at fixed temperature to liquid is called condensation.”
Example: When water vapours at 100°C are cooled they change into water (liquid).
SUBLIMATION : “The process of change of solid directly into vapours on heating is called sublimation.”
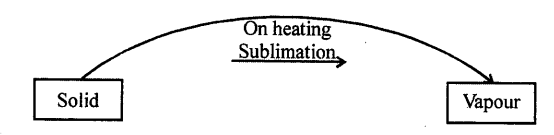
Question 10.
Explain the term melting and melting point.
Answer:
Melting — The change from the solid state to the liquid state on heating at a fixed temperature is called melting.
Melting Point — It can be defined as the fixed temperature at which a solid starts changing to its liquid state.
Question 11.
Describe an experiment to demonstrate that a substance absorbs heat during melting without change in its temperature.
Answer:
MELTING POINT OF SOLID (WAX): Put some wax in a test tube. Insert a thermometer in solid wax, so that bulb of thermometer remains in wax and does not touch the sides. Clamp the test tube along with thermometer in hot bath i.e. in water contained in the beaker and set up the apparatus as shown. Note the temperature Heat the beaker over the flame of burner and record the temperature after every minute. First temperature rises and then reaches 55 °C and wax shines in the test tube. Temperature remains constant for nearly 5 minutes i.e. at 55 °C. This means Wax is melting and temperature remains constant till whole of wax is melted. Then temperature rises again every minute till it reaches
Conclusion : The temperature remains constant at 55°C while changing from solid to liquid. This means 55°C is the melting point and heat is absorbed without change in temperature. This heat is absorbed at constant temperature till whole of wax is melted.
Question 12.
Explain the terms vaporization and boiling point.
Answer:
VAPORIZATION: “Change of liquid to vapours (gas) on heating at constant temperature is called VAPORIZATION.”
When we heat a liquid temperature starts rising till it starts changing into vapours and then temperature remains constant for sometime, through we are supplying heat. This heat supplied is being used to change every molecule of liquid into vapours and temperature does not rise till the whole of liquid is changed into vapours.
BOILING POINT : “The temperature at which a liquid starts changing into vapours or gas at constant temperature is called its BOILING POINT.”
Question 13.
A liquid can change into vapour state
(a) at a fixed temperature, and
(b) at all temperatures
Name the processes involved in two cases.
Answer:
(a) is Boiling point
(b) is Evaporation.
The process involved in two’cases is vaporization or boiling.
Question 14.
Some ice is taken in a beaker and its temperature is recorded after each one minute. The observations are listed below
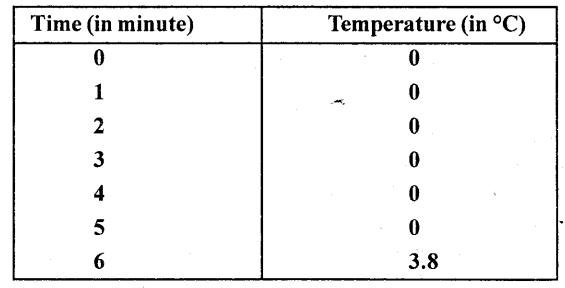
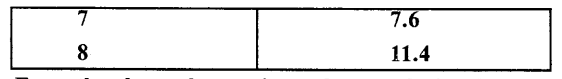
From the above observations what conclusion do you draw about the melting point of ice ?
Answer:
From the above observations we conclude that ice melts at 0°C during which heat is supplied but temperature does not rise shows that heat supplied is used to change every molecule of ice into water and when whole of ice is melted, temperature starts rising.
Question 15.
Describe an experiment to demonstrate that water absorbs heat during boiling at a constant temperature.
Answer:
BOILING POINT OF WATER AT CONSTANT TEMPERATURE:
Take some water in a beaker. Suspend and clamp a thermometer in beaker in water so that bulb of thermometer remains in water without touching bottom and sides of beaker. Supply heat by burner and note the temperature at room temperature (20°C nearly)
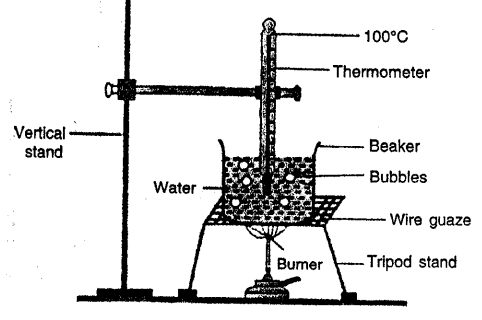
Record the temperature after evefy minute. Temperature rises and as it reaches 100°C water starts boiling. Though heat is being supplied temperature does not rise i.e. it remains constant at 100°C and bubles formed are seen. Thus, boiling point of water is 100°C and at boiling point heat supplied is absorbed by water at constant temperature. Because this heat is being used to change every molecule of water into vapours
Question 16.
State (a) the melting point of ice, and (b) the boiling point of water.
Answer:
(a) MELTING POINT OF ICE: “Is the constant temperature at which it starts (melting) changing from ice to water.”
It is 0°C for ice.
(b) BOILING POINT OF WATER : “Is that constant temperature at which water starts (BOILING) changing from water to steam (vapours)”.
It is 100°C for water.
Question 17.
What is evaporation ?
Answer:
EVAPORATION : “The change of state of a liquid to vapour at all temperatures from the surface of liquid is called evaporation.”
Question 18.
State three factors which affect the rate of evaporation of a liquid.
Answer:
Three factors on which affect the rate of evaporation of a liquid:
(i) AREA OF EXPOSED SURFACE.
(ii) TEMPERATURE OF LIQUID.
(iii) NATURE OF THE LIQUID.
(iv) PRESENCE OF HUMIDITY.
Question 19.
Wet clothes dry more quickly on a warm dry day than on a cold humid day. Explain.
Answer:
Rate of evaporation is directly proportional to temperature. Thus, rate of evaporation is higher on warm day i.e. hot day than cold day having low temperature and clothes dry soon on warm day.
Question 20.
Water in a dish evaporates fasterjhan in a bottle. Give reason.
Answer:
Rate of evaporation is more when the area of exposed surface is more. As area exposed in a dish is more, evaporation is also more.
Question 21.
Why are volatile liquids such as alcohol and spirit stored in tightly closed bottles ?
Answer:
Rate of evaporation depends on NATURE OF LIQUID i.e. more volatile liquids like ALCOHOL and SPIRIT evaporate easily, hence these are stored in tightly closed bottles to avoid their evaporation.
Question 22.
A certain quantity of water is heated from 20°C to 100°C. Its temperature is recorded after each 1 minute. The observations are:
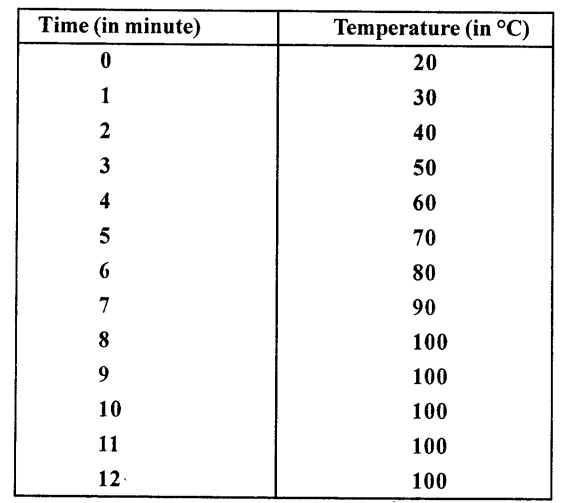
What conclusion do you draw from the above table about the boiling point of water ? Explain.
Answer:
From the table given above we note that as thermometer shows 100°C, it becomes constant and through heat is being supplied. This means boiling point of water is 100°C and heat supplied is being used to convert every molecule of water into vapours (steam) till whole of the water gets boiled off.
Question 23.
Why is cooling produced on evaporation of a liquid ?
Answer:
For evaporation of a liquid it requires HEAT. This heat is taken from the surroundings like body or palm or fore-head or finger and its temperature falls and we feel cool.
Question 24.
Explain with an example to demonstrates that when a liquid
evaporates, it takes heat from its surroundings.
Answer:
If some spirit is poured on cotton wrapped around the bulb of a thermometer, the reading of the thermometer falls. This shows that cooling is produced when a liquid evaporates taking heat from surroundings.
Question 25.
Give two applications of evaporation.
Answer:
Two APPLICATIONS OF EVAPORATION:
(i) When we sprinkle water on the roads in summer evening, water evaporates by taking heat from the road and produces coolness in the surroundings and it becomes pleasant.
(ii) After taking a bath in summer when we come out of water, water evaporates taking heat from our body. The temperature of body falls and we feel refreshed.
Question 26.
Explain why in hot summer days water remains cool in earthen pots.
Answer:
Water seeps out through the pores in the earthen pot and it evaporates. The latent heat required for evaporation is taken, from water inside the~pot which gets cooled.
Question 27.
A patient suffering from high fever is advised to put wet clot strips on his forehead. Why ?
Answer:
Water in wet’ strips evaporates taking latent heat required for evaporation from the forehead. The temperature of forehead (body of the patient) falls and he feels relieved.
Question 28.
What do you mean by sublimation ? Explain with an example.
Answer:
SUBLIMATION : “Change of solid state of matter directly on heating to vapour state (without becoming liquid) and on cooling vapours to solid is called sublimation
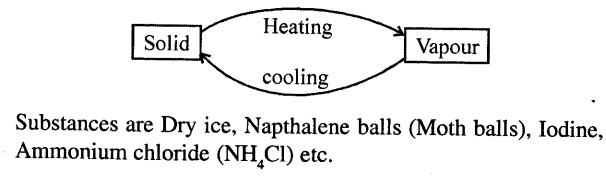
Question 29.
Why does the size of naphthalene balls decrease when left open ?
Answer:
When naphthalene balls are left open, due to sublimation they change to vapours and their size decreases.
Question 30.
Describe an experiment to demonstrate the process of sublimation.
Answer:
Experiment: Take some Ammonium chloride powder in a china dish. Cover the china dish with inverted funnel and put a cotton plug in end of funnel so that vapours do not escape. Set up the apparatus as shown. Heat the dish with burner. Solid ammonium chloride changes into vapours. Which when come in contact of walls of funnel get cooled and change to solid and get deposited there.