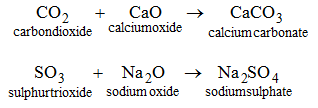
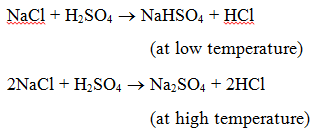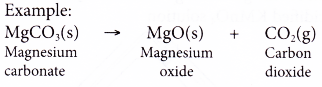Uses of different salts in daily life
- Many different types of salts can be found in nature.
- The sea contains many salts such as sodium chloride, potassium chloride, magnesium chloride, magnesium sulphate and potassium bromide.
- The earth’s crust is made up of minerals containing various types of salts such as calcium fluoride (fluorite), magnesium sulphate (Epsom salt), lead(II) sulphide (galena) and calcium carbonate (limestone).
Uses of salts
- Salts play an important role in our daily life.
- The following gives examples of salts and their uses in daily life.
Agriculture
- Chemical fertilisers
(a) Most of the chemical fertilisers used by farmers are salts.
(b) These salts include ammonium chloride, ammonium nitrate, ammonium phosphate, potassium chloride and NPK fertilisers.
(c) NPK fertilisers contain the elements nitrogen, phosphorus and potassium obtained from salts such as ammonium phosphate and potassium chloride. - Pesticides
(a) Certain salts are used as pesticides to kill or destroy insects, pests, weeds and fungi.
(b) Examples include copper(II) sulphate, iron(II) sulphate, mercury chloride, sodium arsenate and sodium chlorate(V).
Medical field
- Hydrated calcium sulphate, CaSO4.2H2O, is found in plaster of Paris. It is used to make plaster casts for supporting broken bones.
- Iron(II) sulphate heptahydrate, FeSO4.7H2O is an ingredient in ‘iron pills’. It is used as an iron supplement for patients suffering from anaemia.
- Potassium chloride is used as a substitute for patients who need a low intake of sodium salt.
- Magnesium sulphate heptahydrate (Epsom salt) and sodium sulphate decahydrate (Glauber salt) are used as laxatives.
- Sodium hydrogen carbonate is an ingredient in anti-acids. This salt can neutralise the excess acid secreted by the stomach.
- Barium sulphate is used to make barium meals for patients who need to take an X-ray of their stomach. The salt helps to make internal soft organs like intestines appear on X-ray films.
- Potassium manganate(VII) can kill bacteria and hence is suitable for use as an disinfectant.
People also ask
- Classification of Salts
- General Properties of Salts
- Preparation of Salts
- Describe the preparation of soluble and insoluble salts
- Qualitative Analysis of Salts
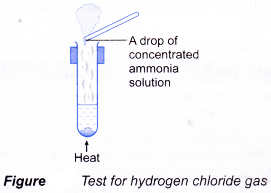
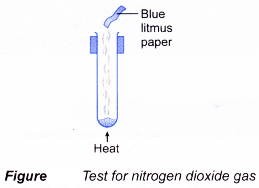
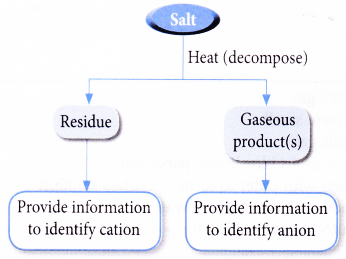
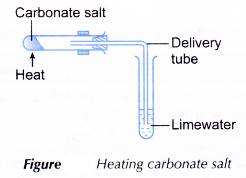
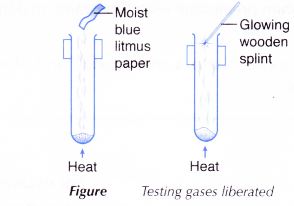
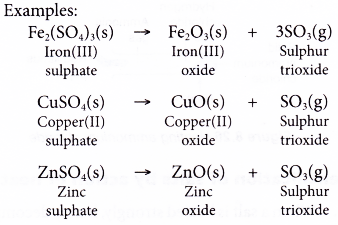
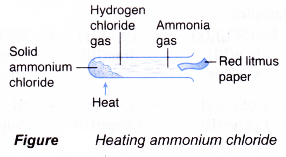
- Action of Heat on Salts
- Test for Cations and Anions in Aqueous Solutions
- Constructing ionic equations using the continuous variation method
- What is stoichiometry and why is it used in chemistry?
Food industry
- Sodium chloride is used as a food additive in the preparation of food, to give foods a salty taste. It is also used as a food preservative.
- Monosodium glutamate (MSG) is another food additive used in food preparation to enhance the taste of foods.
- Sodium hydrogen carbonate is found in baking powders. Together with acid salts such as potassium hydrogen tartrate, calcium hydrogen phosphate and sodium hydrogen phosphate, they react in the presence of water to produce carbon dioxide. As the carbon dioxide escapes, it helps to make the cake or bread rise.
- Sodium nitrite and sodium benzoate are examples of salts used as food preservatives. Sodium nitrite helps to preserve processed meat such as ham and sausages. Sodium benzoate is found in tomato sauce and chilli sauce.
Industry
- Sodium hypochlorite is a bleaching agent and a disinfectant. Cleaning agents contain this compound.
- Tin(II) fluoride is added to toothpaste and water to prevent tooth decay.
- Sodium carbonate decahydrate is used in making soda-lime glass.
- Silver bromide is used in the making of photographic paper and film.
The following table lists uses of some salts:
| Salts | Uses |
| Sodium chloride (NaCl) |
|
| Sodium carbonate (Na2CO3) |
|
| Sodium bicarbonate (NaHCO3) |
|
| Potassium nitrate (KNO3) |
|
| Copper sulphate (CuSO4) |
|
| Potash alum (K2SO4.Al2(SO4)3.24H2O) |
|
| Calcium carbonate (CaCO3) |
|
| Silver nitrate (AgNO3) |
|
| Ammonium nitrate (NH4NO3) |
|