Test for Cations and Anions in Aqueous Solutions
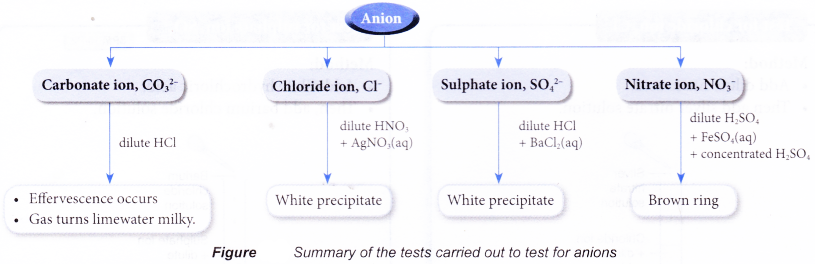
Test for anions in aqueous solutions
- When a salt is dissolved in water, the free anion will be present in the aqueous solution. Tests can then be carried out to identify the anion.
- The following shows the various confirmatory tests for carbonate ion, chloride ion, sulphate ion and nitrate ion in aqueous solutions.
Test for carbonate ion, CO32-
Method:
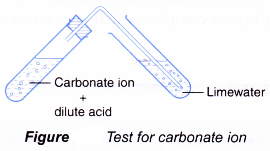
- Add dilute hydrochloric acid.
- Bubble gas through limewater.
Observation:
- Limewater turns milky.
What happened?
- Acids react with carbonate ion to produce carbon dioxide gas.
CO32-(aq) + 2H+(aq) → CO2(g) + H2O(l) - Carbon dioxide turns limewater milky due to the formation of calcium carbonate (white precipitate).
CO2(g) + Ca(OH)2(aq) → CaCO3(s) + H2O(l)
Test for chloride ion, Cl–
Method:
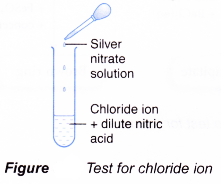
- Add dilute nitric acid.
- Then add silver nitrate solution.
Observation:
- A white precipitate is obtained.
What happened?
- Silver ion, Ag+, from silver nitrate combines with chloride ion, Cl–, to form the silver chloride.
Ag+(aq) + Cl–(aq) → AgCl(s) - Silver chloride is an insoluble salt and forms as a white precipitate.
- The nitric acid added is to prevent precipitation of silver sulphate and silver carbonate.
Test for sulphate ion, SO42-
Method:
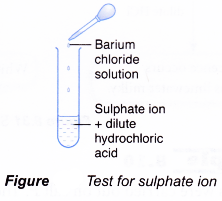
- Add dilute hydrochloric acid.
- Then, add barium chloride solution.
Observation:
- A white precipitate is obtained.
What happened?
- Barium ion, Ba2+, from barium chloride combines with the sulphate ion, SO42-, to form barium sulphate.
Ba2+(aq) + SO42-(aq) → BaSO4(s) - Barium sulphate is an insoluble salt and forms as a white precipitate.
- The hydrochloric acid added is to prevent precipitation of barium carbonate.
Test for nitrate ion, NO3–
Method:
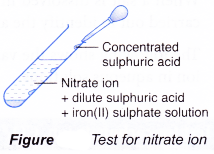
- Add dilute sulphuric acid.
- Then, add iron(II) sulphate solution.
- Shake to mix well.
- Carefully add concentrated sulphuric acid down the side of the test tube.
Observation:
- Brown ring is obtained.
What happened?
- Concentrated sulphuric acid reacts with the nitrate ion to form nitrogen monoxide molecule, NO.
- Nitrogen monoxide combines with iron(II) sulphate to form a brown complex which appears as a brown ring.
- This test is also known as the ‘brown ring test’.
People also ask
- Classification of Salts
- General Properties of Salts
- Uses of different salts in daily life
- Preparation of Salts
- Describe the preparation of soluble and insoluble salts
- Qualitative Analysis of Salts
- Action of Heat on Salts
- Constructing ionic equations using the continuous variation method
- What is stoichiometry and why is it used in chemistry?
Test for anions examples
1. Some tests were carried out on salt P. The results obtained were shown below.
| Test | Observation |
| 1. Salt P was heated. | No residue was left in the test tube. A gas was evolved which turned red litmus paper blue. |
| 2. Salt P was dissolved in water. Dilute nitric acid was added, followed by silver nitrate solution. | A white precipitate was formed. |
Identify salt P.
Solution:
The gas liberated is ammonia because it is an alkaline gas. Hence, ammonium ion is present.
Anion is a chloride ion because silver chloride is precipitated. Salt P is ammonium chloride.
2. Figure shows the reaction scheme of a compound Q.
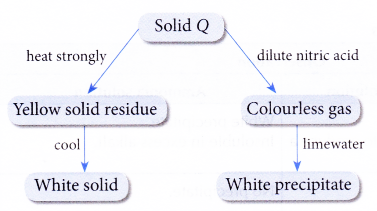
Identify compound Q.
Solution:
The gas is carbon dioxide. Q contains a carbonate ion. Zinc oxide is yellow when hot and white when cold. Hence, Q is zinc carbonate.
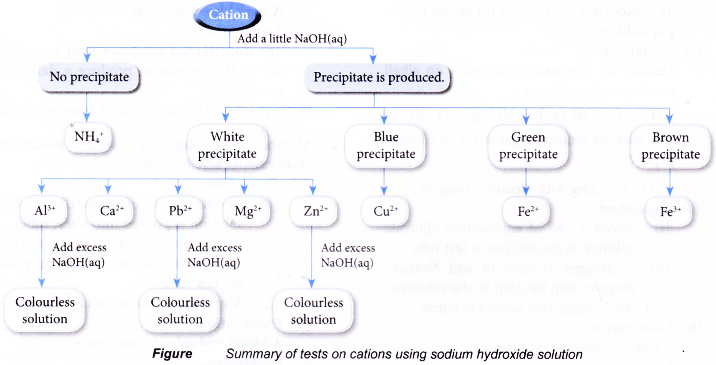
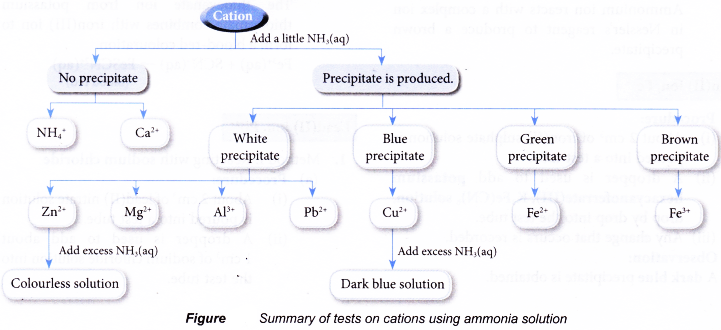
Test for cations in aqueous solutions
- Test for the presence of some common cations such as:
- ammonium ion, NH4+
- aluminium ion, Al3+
- calcium ion, Ca2+
- lead(II) ion, Pb2+
- magnesium ion, Mg2+
- copper(II) ion, Cu2+
- iron(II) ion, Fe2+
- iron(III) ion, Fe3+
- zinc ion, Zn2+
- Aqueous solutions containing the above cations can be prepared by
(a) dissolving a soluble salt in water.
(b) dissolving an insoluble base in dilute acids. - Except for ammonium ion, the rest of the cations in the list are metal ions. They combine with hydroxide ions to form insoluble metal hydroxide.
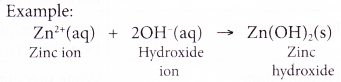
- Two common laboratory reagents that can supply the hydroxide ions needed to test for cations are:
(a) sodium hydroxide solution
(b) ammonia solution
Test for cations in aqueous solutions experiment
Aim: To test for metal cations in aqueous solution.
Materials: 1 mol dm-3 solutions of aluminium nitrate, ammonium chloride, magnesium nitrate, calcium nitrate, lead(II) nitrate, zinc nitrate, iron(II) sulphate, iron(III) chloride, copper(II) sulphate; 2.0 mol dm-3 sodium hydroxide solution, 2.0 mol dm-3 ammonia solution and red litmus paper.
Apparatus: Test tubes, beakers and dropper.
Procedure:
A. Using sodium hydroxide solution to test for metal cations
- About 2 cm3 of aluminium nitrate solution is poured into a test tube.
- A dropper is used to add sodium hydroxide solution drop by drop to the solution in the test tube. The mixture is shaken after each addition of alkali.
- Any changes that occur are noted.
- If a precipitate is produced, the addition of sodium hydroxide solution is continued until in excess. The mixture is shaken well after each addition of alkali.
- Observation on whether the precipitate dissolves in excess alkali is noted.
- Steps 1 to 5 are repeated using each of the cation solutions listed in Table to replace aluminium nitrate solution.
B. Using ammonia solution to test for metal cations
- Steps 1 to 6 in section A are repeated using ammonia solution to replace sodium hydroxide solution.
- The results are recorded in a table.
Observations:
| Cation solution | Cation | Observation | |
| Sodium hydroxide solution | Ammonia solution | ||
| Aluminium nitrate | Al3+ | White precipitate. Dissolves in excess alkali to produce a colourless solution. | White precipitate. Insoluble in excess alkali. |
| Calcium nitrate | Ca2+ | White precipitate. Insoluble in excess alkali. | No precipitate. |
| Copper(II) sulphate | Cu2+ | Blue precipitate. Insoluble in excess alkali. | Blue precipitate. Dissolves in excess alkali to produce a dark blue solution. |
| Iron(II) sulphate | Fe2+ | Green precipitate. Insoluble in excess alkali. | Green precipitate. Insoluble in excess alkali. |
| Iron(III) chloride | Fe3+ | Brown precipitate. Insoluble in excess alkali. | Brown precipitate. Insoluble in excess alkali. |
| Lead(II) nitrate | Pb2+ | White precipitate. Dissolves in excess alkali to produce a colourless solution. | White precipitate. Insoluble in excess alkali. |
| Magnesium nitrate | Mg2+ | White precipitate. Insoluble in excess alkali. | White precipitate. Insoluble in excess alkali. |
| Zinc nitrate | Zn2+ | White precipitate. Dissolves in excess alkali to produce a colourless solution. | White precipitate. |
| Ammonium chloride | NH4+ | No precipitate. | No precipitate. |
Discussion:
- Sodium hydroxide solution is a strong alkali, providing a high concentration of hydroxide ions. Hence, it is able to precipitate all the metal cations used.
- The weaker ammonia solution is unable to ionise fully to provide a high concentration of hydroxide ions needed to precipitate calcium ions.
- Transition metal cations form coloured precipitate, whereas non-transition metal cations form white precipitate.
- Aluminium hydroxide, lead(ll) hydroxide and zinc hydroxide dissolve in excess sodium hydroxide solution due to their amphoteric property, that is, they exhibit both acidic and basic properties.
- Copper(II) hydroxide and zinc hydroxide dissolve in excess ammonia solution because they are able to form complex ions with ammonia molecules.
Conclusion:
Most metal ions can be precipitated in the form of metal hydroxides.
Confirmatory tests for Fe2+, Fe3+, Pb2+ and NH4+ ions
Ammonium ion, NH4+
Method I: Heating an ammonium salt with a strong alkali
(a) Procedure:
(i) About 2 cm3 of ammonium chloride solution is poured into a test tube.
(ii) About 4 cm3 of dilute sodium hydroxide solution is added to the test tube and the mixture is shaken well.
(iii) The mixture is carefully heated and the gas liberated is tested with a piece of moist red litmus paper.
(b) Observation:
The colourless gas evolved turns red litmus paper blue.
(c) Explanation:
Heating an ammonium salt with an alkali produces ammonia gas.
NH4+(aq) + OH–(aq) → NH3(g) + H2O(l)
The alkaline ammonia gas turns red litmus paper blue.
Method II: Reacting with Nessler’s reagent
(a) Procedure:
(i) About 2 cm3 of ammonium chloride solution is poured into a test tube.
(ii) A dropper is used to add Nessler’s reagent drop by drop to the solution.
(iii) Any change that occurs is noted.
(b) Observation:
A brown precipitate is formed.
(c) Explanation:
Ammonium ion reacts with a complex ion in Nessler’s reagent to produce a brown precipitate.
Iron(II) ion, Fe2+
(a) Procedure:
(i) About 2 cm3 of iron(II) sulphate solution is poured into a test tube.
(ii) A dropper is used to add potassium hexacyanoferrate(III), K3Fe(CN)6 solution, drop by drop into the test tube.
(iii) Any change that occurs is recorded.
(b) Observation:
A dark blue precipitate is obtained.
(c) Explanation:
The iron(II) ion combines with a complex ion in the reagent to produce a dark blue precipitate.
Fe2+(aq) + Fe(CN)63-(aq) → dark blue precipitate
Iron(III) ion, Fe3+
Method I: Reacting with potassium hexacyanoferrate(II), K4Fe(CN)6 solution
(a) Procedure:
(i) About 2 cm3 of iron(III) chloride solution is poured into a test tube.
(ii) A dropper is used to add potassium hexacyanoferrate(II) solution drop by drop into the test tube.
(iii) Any change that occurs is noted.
(b) Observation:
A dark blue precipitate is formed.
(c) Explanation:
The iron(III) ion combines with a complex ion in the reagent to produce a dark blue precipitate.
Fe3+(aq) + Fe(CN)64+(aq) → dark blue precipitate
Method II: Reacting with potassium thiocyanate, KSCN solution
(a) Procedure:
(i) About 2 cm3 of iron(III) chloride solution is poured into a test tube.
(ii) A dropper is used to add potassium thiocyanate solution drop by drop into the test tube.
(iii) Any change that occurs is recorded.
(b) Observation:
A blood-red colouration is seen.
(c) Explanation:
The thiocyanate ion from potassium thiocyanate combines with iron(III) ion to form a blood-red colouration.
Fe3+(aq) + SCN–(aq) → FeSCN2+(aq) (blood red)
Lead(II) ion, Pb2+
Method I: Reacting with sodium chloride
(a) Procedure:
(i) About 2 cm3 of lead(II) nitrate solution is poured into a test tube.
(ii) A dropper is used to add about 1 cm3 of sodium chloride solution into the test tube.
(iii) About 3 cm3 of distilled water is added and the mixture is boiled.
(iv) The mixture is then cooled using running water from the tap.
(v) Any change that occurs is recorded.
(b) Observation:
A white precipitate which dissolves in hot water and reappears when cooled is formed.
(c) Explanation:
Chloride ion from sodium chloride combines with lead(II) ion to form a white precipitate of lead(II) chloride.
Pb2+(aq) + 2Cl–(aq) → PbCl2(s)
The precipitate is insoluble in cold water but dissolves in hot water.
Method II: Reacting with potassium iodide
(a) Procedure:
(i) About 2 cm3 of lead(II) nitrate solution is poured into a test tube.
(ii) A dropper is used to add about 1 cm3 of potassium iodide solution into the test tube.
(iii) About 3 cm3 of distilled water is added and the mixture is boiled.
(iv) The mixture is then cooled using running water from the tap.
(v) Any change that occurs is recorded.
(b) Observation:
A yellow precipitate which dissolves in hot water is formed.
On cooling, golden yellow crystals are formed.
(c) Explanation:
Iodide ion from potassium iodide combines with lead(II) ion to form a yellow precipitate of lead(II) iodide.
Pb2+(aq) + 2I–(aq) → PbI2(s)
The precipitate is insoluble in cold water but dissolves in hot water.
| Cation | Sodium hydroxide solution | Ammonia solution | Other reagent |
NH4+ | Ammonia gas evolved when mixture is heated. | – | Nessler’s reagent: brown precipitate |
| Mg2+ | White precipitate. Insoluble in excess alkali. | White precipitate. Insoluble in excess alkali. | – |
| Ca2+ | White precipitate. Insoluble in excess alkali. | No precipitate. | Concentrated H2SO4: white precipitate |
| Pb2+ | White precipitate. Soluble in excess alkali to form colourless solution. | White precipitate. Insoluble in excess alkali. | Kl(aq): yellow precipitate NaCl(aq): white precipitate Na2S04(aq): white precipitate |
| Al3+ | White precipitate. Soluble in excess alkali to form colourless solution. | White precipitate. Insoluble in excess alkali. | – |
| Zn2+ | White precipitate. Soluble in excess alkali to form colourless solution. | White precipitate. Soluble in excess alkali to form colourless solution. | – |
| Fe2+ | Green precipitate. Insoluble in excess alkali. | Green precipitate. Insoluble in excess alkali. | K3Fe(CN)6: dark blue precipitate KMn04: purple colour of the solution is decolourised |
| Fe3+ | Brown precipitate. Insoluble in excess alkali. | Brown precipitate. Insoluble in excess alkali. | K4Fe(CN)K6: dark blue precipitate KSCN: blood-red colouration |
| Cu2+ | Blue precipitate. Insoluble in excess alkali. | Blue precipitate. Soluble in excess alkali to form dark blue solution. | – |
Identifying the anions and cations in unknown salts
- Use the knowledge you have- learnt about the reactions of anions and cations to help you plan and carry out experiments to identify the anions and cations in an unknown salt.
- This knowledge can also help you write the correct observations and make inferences or conclusions about the identities of anions and cations.
- When carrying out tests on a salt:
(a) plan your experiment carefully
(b) be systematic and meticulous
(c) use correct techniques
(d) always follow safety procedures - The salt is dissolved in water or dilute acid.
- The first step in qualitative analysis of a salt is to obtain an aqueous solution of the given salt.
- A soluble salt will dissolve in water to produce ions in aqueous solution.
- Insoluble salts such as an insoluble carbonate can be dissolved in dilute nitric acid to produce ions in aqueous solutions.
Identifying the anions and cations examples
1. Q1 is a simple salt. Carry out the following tests to identify the salt.
| Experiment | Observation | Deduction |
|
|
|
|
|
|
|
|
|
Salt Q1 contains lead(II) ion, Pb2+, and nitrate ion, NO3+. Q1 is lead(II) nitrate.
2. Q2 is a salt containing one cation and one anion. Identify the ions from the following tests.
| Experiment | Observation | Deduction |
|
|
|
|
|
|
Q2 is iron(III) carbonate as it contains iron(III) ion, Fe3+, and carbonate ion, CO32- .