Physical Properties Of Metals And Nonmetals
Different elements have different properties. These properties can make these elements suitable for various purposes. Elements can be classified into two broad categories: metals and non-metals. Aluminium and mercury are examples of metals. Diamond and oxygen are examples of non-metals.
Let us learn about physical and chemical properties of metals and non-metals.
Physical properties include physical state, lustre, colour, hardness, malleability, ductility, thermal conductivity, electrical conductivity, and sonority.
Physical State
Metals: Almost all metals are solids at room temperature. Mercury, gallium, francium, caesium, and rubidium are the only metals known to occur in a liquid state at or near room temperature.
Non-metals: Almost all non-metals are solids or gases at room temperature. Bromine is the only non-metal that exists as a liquid at room temperature.
Lustre
Metals: ‘Glitter’ or a shiny surface is a property of most metals. This is because metals can be polished. This property is called lustre. Because of their ability to shine and reflect light, metals like gold, silver, and platinum are used for making jewellery and other decorative articles.
Non-metals: Almost all non-metals have a dull surface. As most of them occur as powders and gases, they cannot be polished like metals. Graphite and iodine do show some lustre.

Colour
Metals: Most metals are white or silvery-grey. There are, however, a few exceptions. For example, gold is yellow and copper is reddish-brown.
Non-metals: Some non-metals are colourless while some are coloured. For example, chlorine is a greenish-yellow gas, bromine is a brown liquid, iodine is a violet solid, and oxygen and nitrogen are colourless gases.
Hardness
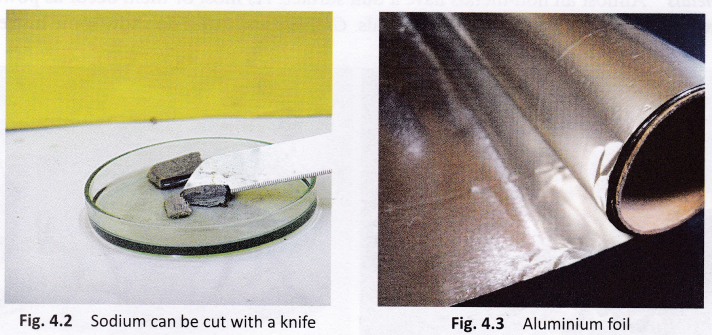
Metals: Most metals are hard but some (e.g., sodium and potassium) are so soft that they can be cut with a knife.
Non-metals: Non-metals are generally soft. Diamond is an exception. It is the hardest substance known.
Malleability
Metals: Most metals can be beaten into thin sheets or foils. The property by virtue of which metals can be beaten into thin sheets is called malleability. Gold and silver are the most malleable metals known. This is what helps jewellery designers create intricately carved bangles, chains, and decorative articles in gold and silver. Other metals that can be beaten into sheets include aluminium, iron, copper, and tin.
Non-metals: Non-metals are brittle and cannot be beaten into sheets or foils.
Activity
Aim: To show that metals are malleable and non-metals are brittle.
Materials needed: Small samples of easily available metals (e.g., aluminium wire, iron nail, copper wire, etc.) and non-metals (e.g., graphite and charcoal) and a hammer.
Method: Pound the objects one by one with the help of the hammer.
Observation: Metal objects get flattened upon hammering while non-metals break.
Conclusion: Metals are malleable whereas non-metals are brittle.
Note: Adult supervision required.
Ductility
Metals: Most metals can easily be drawn into thin wires, which have a wide range of applications. The property by virtue of which metals can be drawn into thin wires is called ductility. Gold and silver are two of the most ductile metals known. Other metals that can be drawn into wires include copper, aluminium, and tungsten.
Non-metals: Non-metals are brittle and cannot be drawn into wires.
Thermal Conductivity
Metals: Metals are good conductors of heat and are, therefore, used for making cooking utensils. Silver is the best conductor of heat followed by copper.
Non-metals: Non-metals are generally poor conductors of heat. Diamond, which is a good conductor of heat, is an exception.
Electrical Conductivity
Metals: Metals are good conductors of electricity and are, therefore, used for making electrical wires and cables.
Non-metals: Non-metals are generally poor conductors of electricity. Graphite, which is a good conductor of electricity, is an exception.
Activity
Aim: To show that metals and graphite are good conductors of electricity and other non-metals are poor conductors.
Materials needed: Small samples of easily available metals (e.g., aluminium wire, iron nail, copper wire, etc.) and non-metals (e.g., graphite and charcoal), a copper wire cut into three pieces, a pencil cell, and a 1.5-volt bulb.
Method:
1. Set up the apparatus as shown in the figure.
2. Connect the two free ends (A and B) of the copper wire to the objects, one by one.
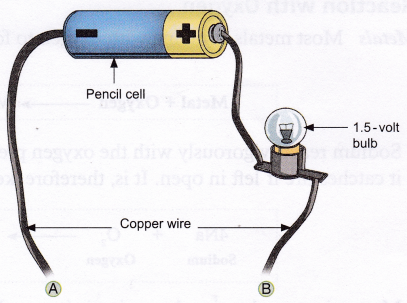
Observation: The bulb glows when metals and graphite are connected to the free ends of the copper wire, but not for other non-metals.
Conclusion: Metals and graphite are good conductors of electricity whereas non-metals are poor conductors.
Sonority
Metals: When metal pipes strike each other, they produce a ringing sound. The property by virtue of which metal objects produce a ringing sound when struck with a hard object is called sonority. Objects like wind chimes and bells make use of this property of metals.
Non-metals: Non-metals produce a dull sound when struck with a hard object.