Chemical Properties Of Metals And Nonmetals
Chemical properties of metals and non-metals can be divided into five categories: reaction with oxygen, reaction with water, reaction with acids, reaction with bases, and displacement reactions.
Reaction with Oxygen
Metals: Most metals combine with oxygen to form metal oxides.

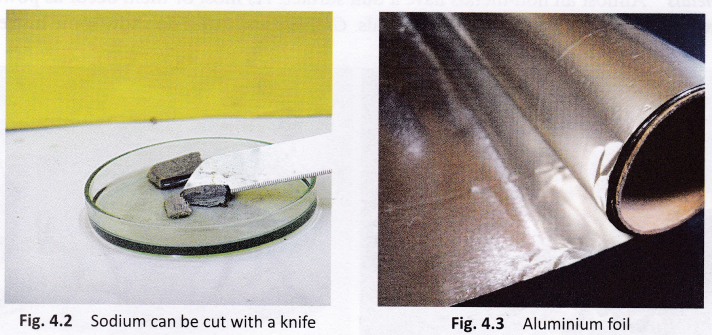
- Sodium reacts vigorously with the oxygen present in air to form sodium oxide. As a result, it catches fire if left in open. It is, therefore, kept immersed in kerosene.
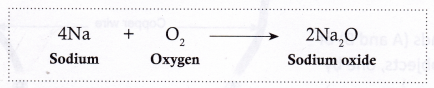
- Magnesium, on heating, burns in air (oxygen) with a dazzling white light to form magnesium oxide.
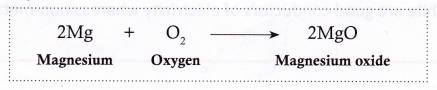
The metallic oxides formed are basic in nature and turn red litmus solution blue.
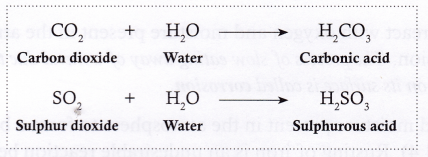
Non-metals: Non-metals like carbon, sulphur, and phosphorus react with oxygen to form non-metallic oxides. These oxides are also called acidic oxides as they form acids when dissolved in water.

- Carbon burns in air (oxygen) to form carbon dioxide.
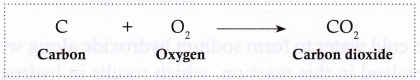
- Sulphur burns in air (oxygen) to form a pungent (i.e., having a strong smell), suffocating gas called sulphur dioxide.
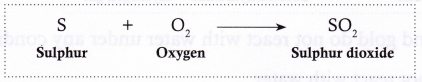
- These oxides dissolve in water to form acids.
Activity
Aim: To synthesize a non-metallic oxide and test its solution using litmus paper.
Materials needed: Sulphur, a long-handled spoon or a deflagrating spoon, burner, water, gas jar with a lid, and blue litmus paper.
Method:
- Take a small amount of sulphur in the long-handled spoon/ deflagrating spoon and heat it over the flame of a burner.
- When sulphur starts burning, lower the spoon into the gas jar. Cover the jar partly with the lid while the sulphur is still burning.
- The jar will be filled with sulphur dioxide gas. Remove the spoon and cover the gas jar with a lid.
- Add 20 ml water to the gas jar and test this solution with blue litmus paper.
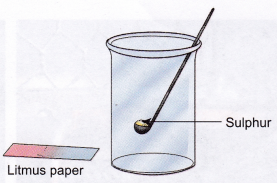 Observation: Blue litmus paper turns red, indicating that the solution is acidic.
Observation: Blue litmus paper turns red, indicating that the solution is acidic.
Conclusion: Water dissolves the gas (sulphur dioxide) to form an acid (sulphurous acid), which turns blue litmus red.
Note: Adult supervision required.
Reaction with Water
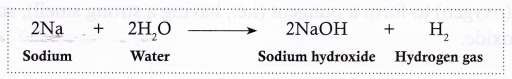
Metals Most metals react with water to produce a metal hydroxide or metal oxide and hydrogen gas.

- Sodium reacts violently with cold water to form sodium hydroxide along with hydrogen gas. A large amount of heat is evolved in this reaction, which results in hydrogen catching fire.
- Metals like copper, silver, and gold do not react with water under any conditions.
Non-metals: Non-metals do not react with water.
Corrosion
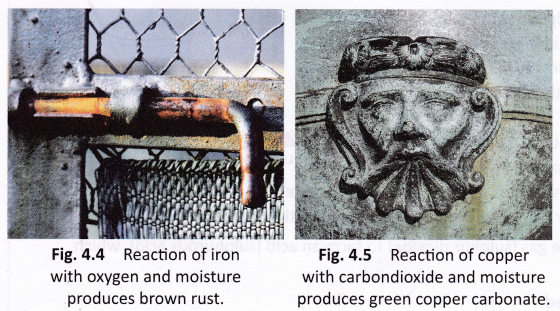
Iron and many other metals react with oxygen and moisture present in the atmosphere. This phenomenon is called corrosion. The process of slow eating away of a metal due to the attack of atmospheric gases and moisture on its surface is called corrosion.
- Iron reacts with oxygen and moisture present in the atmosphere to form a brown, flaky substance called rust. Rusting of iron is an undesirable reaction because the layer of rust formed falls off, exposing the metal to further rusting. As a result, iron objects become weak with the passage of time.
- Copper objects get coated with a green substance called basic copper carbonate with the passage of time. This green substance is formed due to the reaction of copper with carbon dioxide and moisture present in the atmosphere.
- Silver objects become blackened and lose their sheen with the passage of time. This happens due to the reaction of silver with hydrogen sulphide gas present in the atmosphere.

Reaction with Acids
Metals When a metal reacts with an acid, a salt and hydrogen gas are produced.
Salts are compounds formed when a metal replaces hydrogen in an acid. Different acids and metals react to form different salts.

- Zinc reacts with sulphuric acid to form zinc sulphate and hydrogen gas.
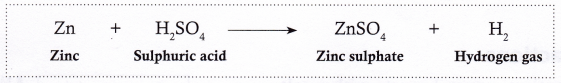
- Magnesium reacts with sulphuric acid to form magnesium sulphate and hydrogen gas.

- Aluminium reacts with hydrochloric acid to form aluminium chloride and hydrogen gas.
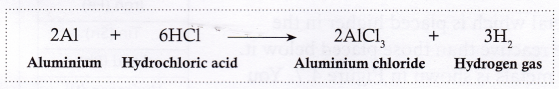
With some metals, the reaction is very fast and vigorous, while with others it may be slow. Some metals do not react with acids at all.
Non-metals: Generally, non-metals do not react with acids.
Activity
Aim: To show that hydrogen is produced when magnesium reacts with dilute sulphuric acid
Materials needed: Magnesium ribbon, dilute sulphuric acid, test tube, dropper, and a matchstick
Method:
- Take a piece of magnesium ribbon in the test tube.
- Using a dropper, carefully add a few drops of dilute sulphuric acid from the sides of the test tube.
- Bring a burning matchstick near the mouth of the test tube.
Observation: Bringing a burning matchstick nearthe mouth ofthe test tube produces a ‘pop’ sound. Conclusion: The gas produced in the reaction is hydrogen.
Note: Adult supervision required.
Reaction with Bases
Metals: Most metals do not react with bases. Only a few, like aluminium, zinc, and lead, react with solutions of strong bases like sodium hydroxide to produce a compound of that metal and hydrogen gas. You could perform the above activity using small pieces of zinc and sodium hydroxide solution (instead of magnesium ribbon and dilute sulphuric acid) to test that hydrogen is evolved in the reaction.
Non-metals: The reactions of non-metals with bases are complex. You will learn about them in higher classes.
Displacement Reactions
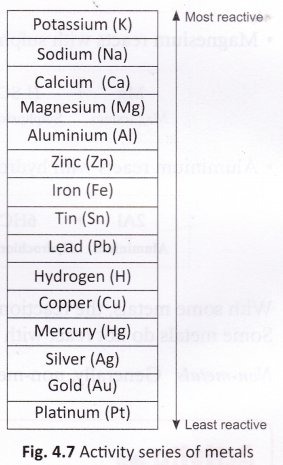 In a displacement reaction, a metal reacts with a salt solution and ‘displaces’ (or replaces) the metal present in it. Displacement reactions are explained on the basis of the activity series of metals.
In a displacement reaction, a metal reacts with a salt solution and ‘displaces’ (or replaces) the metal present in it. Displacement reactions are explained on the basis of the activity series of metals.
The activity series of metals is a list of common metals arranged in the decreasing order of reactivity. This means that a metal which is placed higher in the activity series is more reactive than those placed below it. The activity series of metals is shown in figure. You can predict whether or not a displacement reaction will take place by looking at the activity series. A metal will only react with a salt solution if it is placed higher in the activity series than the metal in the salt. For example, iron, which is placed higher in the activity series than copper, reacts with copper sulphate solution. Copper, however, does not react with iron sulphate as it is less reactive than iron. Some more examples are discussed below.
- Silver does not react with zinc sulphate.

- Zinc reacts with copper sulphate to form zinc sulphate and copper.
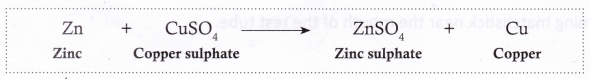
- Silver does not react with copper sulphate.

From the above reactions, we can conclude that the order of reactivity of zinc, copper, and silver is: Zn > Cu > Ag (i.e., zinc is the most reactive of the three and silver, the least reactive).
Activity
Aim: To prove that iron is more reactive than copper.
Materials needed: Iron filings, copper turnings, copper sulphate solution, iron sulphate solution, test tubes, and a dropper.
Method:
- Take some iron filings in a test tube and add some copper sulphate solution with the help of a dropper (test tube A).
- Take some copper turnings in a test tube and add some iron sulphate solution with the help of a dropper (test tube B).
 Observation: In test tube A, iron filings turn brown due to the deposition of copper and the solution turns pale green due to the formation of iron sulphate solution. No reaction is observed in test tube B.
Observation: In test tube A, iron filings turn brown due to the deposition of copper and the solution turns pale green due to the formation of iron sulphate solution. No reaction is observed in test tube B.
Conclusion: Iron is more reactive than copper as it displaces copper from copper sulphate solution.

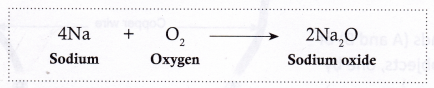
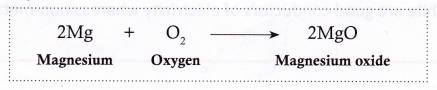

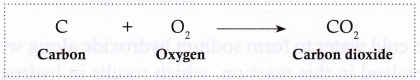
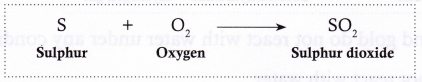

 Observation: Blue litmus paper turns red, indicating that the solution is acidic.
Observation: Blue litmus paper turns red, indicating that the solution is acidic.






 In a displacement reaction, a metal reacts with a salt solution and ‘displaces’ (or replaces) the metal present in it. Displacement reactions are explained on the basis of the activity series of metals.
In a displacement reaction, a metal reacts with a salt solution and ‘displaces’ (or replaces) the metal present in it. Displacement reactions are explained on the basis of the activity series of metals.


 Observation: In test tube A, iron filings turn brown due to the deposition of copper and the solution turns pale green due to the formation of iron sulphate solution. No reaction is observed in test tube B.
Observation: In test tube A, iron filings turn brown due to the deposition of copper and the solution turns pale green due to the formation of iron sulphate solution. No reaction is observed in test tube B.
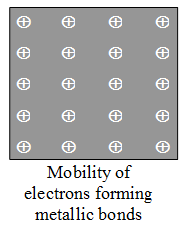 X-rays analysis of metal crystal has revealed that each atom in metal crystal is surrounded by 8 or 12 other metal atoms. In metal atoms, the valency electrons are few (1, 2, and 3) and thus, it is not possible for a metal atom to form 8 to 12 covalent bonds with neighbouring atoms. Thus, it was assumed that the atoms in metal crystal are bonded with each other with a special type of bonding known as metallic bonding. Drude in 1900 proposed the theory of metallic bonding which was later on modified by Lorentz. According to these authors, metals having 1, 2 or 3 electrons in outermost shells, being electropositive lose their electron readily because of low IE values to form free electrons and remainder portion of atom with a Kernel (core of stable nature) carrying positive charge. The free electrons are mobile in nature and move from the one Kernel to another which are closely packed in regular fashion throughout the crystal lattice. thus, the metal crystal is represented by an arrangement of positively charged Kernels in a sea of mobile electrons (Figure) shared by each Kernal to give metallic bonds. Ad the shared electrons are delocalized, the metallic bonds have neither direction nor saturation. There are two essential conditions for metallic bonding :
X-rays analysis of metal crystal has revealed that each atom in metal crystal is surrounded by 8 or 12 other metal atoms. In metal atoms, the valency electrons are few (1, 2, and 3) and thus, it is not possible for a metal atom to form 8 to 12 covalent bonds with neighbouring atoms. Thus, it was assumed that the atoms in metal crystal are bonded with each other with a special type of bonding known as metallic bonding. Drude in 1900 proposed the theory of metallic bonding which was later on modified by Lorentz. According to these authors, metals having 1, 2 or 3 electrons in outermost shells, being electropositive lose their electron readily because of low IE values to form free electrons and remainder portion of atom with a Kernel (core of stable nature) carrying positive charge. The free electrons are mobile in nature and move from the one Kernel to another which are closely packed in regular fashion throughout the crystal lattice. thus, the metal crystal is represented by an arrangement of positively charged Kernels in a sea of mobile electrons (Figure) shared by each Kernal to give metallic bonds. Ad the shared electrons are delocalized, the metallic bonds have neither direction nor saturation. There are two essential conditions for metallic bonding :