What is the Haber process used for?
Manufacture of ammonia in industry
- Most of the world supply of ammonia is manufactured through Haber process.
- The raw materials for the manufacture of ammonia are hydrogen gas and nitrogen gas. The ratio for the raw materials is one portion of nitrogen to three portions of hydrogen.
(a) Nitrogen is obtained from the fractional distillation of liquid air.
(b) Hydrogen can be obtained by two methods: The reaction between methane (from natural gas) or heated coke with steam.
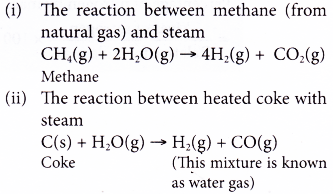
- The optimum conditions for Haber process is
(a) Temperature: 450°C
(b) Pressure: 200 atmosphere
(c) Catalyst: red hot iron - During Haber process:
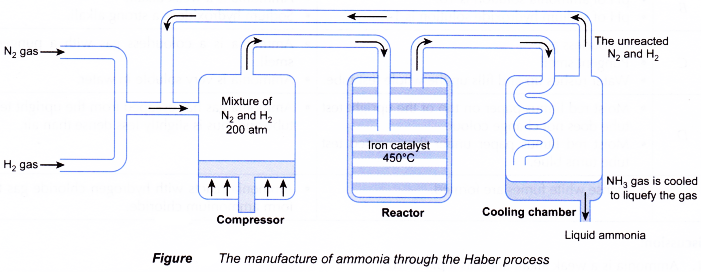
(a) A mixture of one volume of dry nitrogen gas and three volume of pure hydrogen gas are mixed and passed to the compressor and compressed to a pressure of about 200 atm.
(b) The mixture is then passed through layers of heated and finely divided iron catalyst in the reactor at a temperature of about 450°C.
(c) Ammonia is produced in the reactor but the reaction is reversible.
(d) The production of ammonia is exothermic and gives out heat. It is passed into the cooling chamber, then liquefied and separated to get a better yield.
(e) The unreacted nitrogen and hydrogen are recycled and passed back into the reactor converted into ammonia,
together with the new source of nitrogen and hydrogen. - About 98% of nitrogen and hydrogen are converted into ammonia.
People also ask
- What are the physical properties of ammonia?
- Uses of ammonia in our daily life
- How is Sulfuric Acid Made?
- Uses of sulphuric acid in daily life
- How Acid rain is formed equations?
Preparation of ammonium sulphate experiment
Aim: To prepare ammonium sulphate.
Materials: 1 mol dm-3 sulphuric acid, 2 mol dm-3 ammonia solution, methyl orange, filter paper.
Apparatus: 25.0 cm3 pipette, burette, conical flask, white tile, retort stand and clamp, beaker, glass rod, evaporating dish, filter funnel, Bunsen burner, tripod stand, wire gauze.
Procedure:
A. Determining the volume of sulphuric acid that will neutralise 25.0 cm3 of ammonia solution
Safety Measures
- Concentrated sulphuric acid is corrosive.
- Do not inhale ammonia gas.
- 25.0 cm3 of 2 mol dm-3 ammonia solution is measured and transferred by a pipette to a clean conical flask.
- Three drops of methyl orange indicator are added to the alkali. The solution turns yellow.
- A clean burette is filled with 1 mol dm-3 sulphuric acid and clamped to a retort stand.
- The initial burette reading is recorded.
- The conical flask with its contents is placed on a white tile below the burette as shown in Figure.
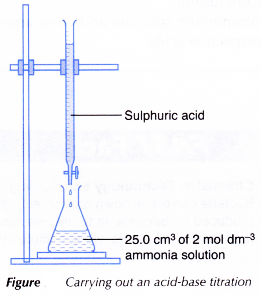
- The sulphuric acid is added slowly into the conical flask. The conical flask is swirled gently throughout the titration.
- The addition of the sulphuric acid is stopped when the indicator changes from yellow to orange.
- The final burette reading is recorded.
- The volume of acid needed to completely neutralise the 25.0 cm3 of 2 mol dm-3 ammonia solution is alculated. Let this volume be V cm3.
B. Preparing ammonium sulphate salt
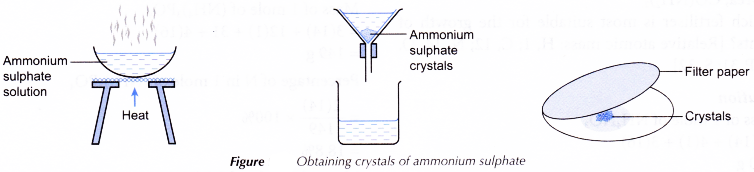
- 25.0 cm3 of 2 mol dm-3 ammonia solution is pipetted into a clean beaker. No indicator is added.
- V cm3 of 1 mol dm-3 sulphuric acid is added from the burette to the ammonia solution and stirred.
- The mixture in the beaker is transferred to an evaporating dish and heated until a saturated solution is formed.
- The hot, saturated salt solution is left to cool for crystallisation to occur.
- The crystals of ammonium sulphate formed are filtered and pressed between sheets of filter paper.
Observations:
- A colourless solution is formed when sulphuric acid is added to ammonia solution.
- The crystals obtained are white in colour.
Discussion:
- The equation for the reaction is
H2SO4(aq) + 2NH3(aq) → (NH4)2SO4(aq) - Methyl orange is an acid-base indicator used to determine the end point of the titration.
- The first titration is carried out to determine the exact volume of sulphuric acid required to completely neutralise the 25.0 cm3 of ammonia solution.
- The salt solution in the first titration is discarded because it is contaminated by methyl orange.
- The ammonium sulphate solution should not be heated until dryness because ammonium sulphate decomposes when it is overheated.
- The weight of ammonium sulphate obtained from the activity is always less than the theoretical value. This is because some of the salt is not fully crystallised out and still remains in the solution.
- Other ammonium fertilisers such as ammonium nitrate can be prepared from the reaction between nitric acid and ammonia solution.
Conclusion:
Ammonium sulphate and other ammonium fertilisers can be prepared by neutralising ammonia solution with the respective acids.