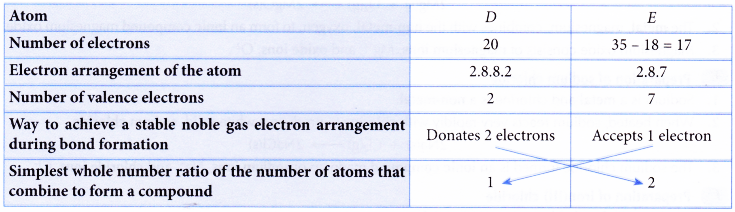
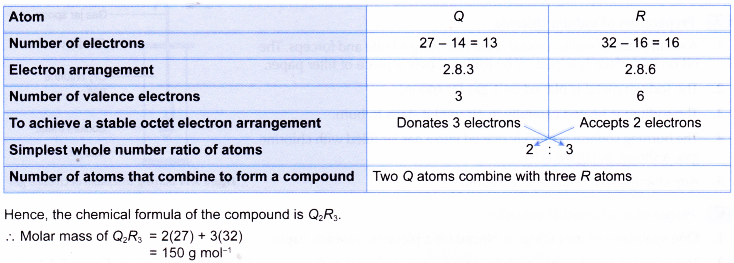
What are the Two Types of Ions and how are they Different

Ion:
An ion is a positively or negatively charged atom (or group of atoms). An ion is formed by the loss or gain of electrons by an atom, so it contains an unequal number of electrons and protons.
Example: Sodium ion Na+, magnesium ion Mg2+, chloride ion Cl–, and oxide ion O2–.
There are two types of ions :
- cations
- anions.

1. Cation
A positively charged ion is known as cation. A cation is formed by the loss of one or more electrons by an atom.
Example: Sodium atom loses 1 electron to form a sodium ion, Na+, which is cation :
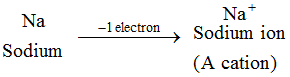 The ions of all the metal elements are cations.
The ions of all the metal elements are cations.
 2. Anion
2. Anion
A negatively charged ion is known as anion. An anion is formed by the gain of one or more electrons by an atom.
Example: A chlorine atom gains 1 electron to form a chloride ion, Cl–, which is an anion.
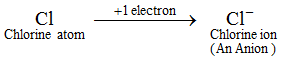 An anion contains more electrons than a normal atom. A normal atom (or a neutral atom) contains an equal number of protons and electrons. Now, since an anion is formed by the addition of one or more electrons to an atom, therefore, an anion contains more electrons than protons. The ions of all the non metal elements are anions.
An anion contains more electrons than a normal atom. A normal atom (or a neutral atom) contains an equal number of protons and electrons. Now, since an anion is formed by the addition of one or more electrons to an atom, therefore, an anion contains more electrons than protons. The ions of all the non metal elements are anions.
 Simple ions
Simple ions
Those ions which are formed from single atoms are called simple ions.
Example: Sodium ion, Na+, is a simple ion because it is formed from a single sodium atom, Na.
Compound ions
Those ions which are formed from groups of joined atoms are called compound ions
Example: Ammonium ion NH4+, is a compound ion which is made up of two types of atoms joined together, nitrogen and hydrogen.
Ionic compounds
The compounds which are made up of ions are known as ionic compounds. In an ionic compound, the positively charged ions (cations) and negatively charged ions (anions) are held together by the strong electrostatic forces of attraction. The forces which hold the ions together in an ionic compound are known as ionic bonds or electrovalent bonds. Since an ionic compound consists of an equal number of positive ions and negative ions, so the overall charge on an ionic compound is zero.
Example: Sodium chloride (NaCl) is an ionic compound which is made up of equal number of positively charged sodium ions (Na+) and negatively charged chloride ions (Cl–).
Some ionic compound
S.No. | Name | Formula | Ions present |
1 | Sodium chloride | NaCl | Na+ and Cl– |
| 2 | Potassium chloride | KCl | K+ and Cl– |
3 | Ammonium chloride | NH4Cl | NH4+ and Cl– |
4 | Magnesium chloride | MgCl2 | Mg2+ and Cl– |
| 5 | Calcium chloride | CaCl2 | Ca2+ and Cl– |
| 6 | Magnesium oxide | MgO | Mg2+ and O2– |
| 7 | Calcium oxide | CaO | Ca2+ and O2– |
| 8 | Aluminium oxide | Al2O3 | Al3+ and O2– |
| 9 | Sodium hydroxide | NaOH | Na+ and OH– |
| 10 | Copper sulphate | CuSO4 | Cu2+ and SO42– |
| 11 | Calcium nitrate | Ca(NO3)2 | Ca2+ and NO3– |