How do you write the formula for ionic compounds?
Method to deduce the formulae of ionic compounds:
Metals from Groups 1, 2 and 13 combine with non-metals from Groups 15, 16 and 17 to form ionic compounds.
Table shows how the metal atoms of Groups 1, 2 and 13 form positive ions with different charges.
| Group | Number of valence electrons | Number of electrons donated to achieve a stable noble gas electron arrangement | Charge of positive ion | Example of ions |
| 1 | 1 | 1 | + 1 | Li+, Na+, K+ |
| 2 | 2 | 2 | +2 | Mg2+, Ca2+, Ba2+ |
| 3 | 3 | 3 | +3 | Al3+ |
Table shows how the non-metal atoms of Groups 15, 16 and 17 form negative ions with different charges.
| Group | Number of valence electrons | Number of electrons accepted to achieve a stable noble gas electron arrangement | Charge of negative ion | Example of ions |
| 15 | 5 | 3 | -3 | N3- |
| 16 | 6 | 2 | -2 | O2-, S2- |
| 17 | 7 | 1 | -1 | F–, Cl–, Br–, I– |
Table shows the formulae of ionic compounds obtained when a metal from Group 1, 2 or 13 combines with a non-metal from Group 15, 16 or 17.
| Elements that combined | Formula of ionic compound | Example | |
| Metal atom R from | Non-metal atom T from | ||
| Group 1 | Group 17 | RT | Potassium chloride, KCl |
| Group 1 | .Group 16 | R2T | Sodium oxide, Na2O |
| Group 1 | Group 15 | R3T | Lithium nitride, Li3N |
| Group 2 | Group 17 | RT2 | Calcium fluoride, CaF2 |
| Group 2 | Group 16 | RT | Magnesium sulphide, MgS |
| Group 2 | Group 15 | R3T2 | Calcium nitride, Ca3N2 |
| Group 13 | Group 17 | RT3 | Aluminium chloride, AICI3 |
| Group 13 | Group 16 | R2T3 | Aluminium oxide, Al2O3 |
| Group 13 | Group 15 | RT | Aluminium nitride, AlN |
The information in above Tables can be used to determine the formulae of ionic compounds as shown in the following examples.
People also ask
- Chemical Bonding and Compound Formation
- Chemical Bonding
- What is Covalent Bond?
- How is covalent bond is formed?
- Describe how to write a formula for a covalent compound
- What causes ions to form ionic bonds?
- Explain the formation of ionic bonds with examples
- Properties of Ionic and Covalent Compounds
- How do you Name an Ionic Compound?
Writing formulas for ionic compounds examples
1. An atom of element D has 20 electrons. An atom of element E has 18 neutrons and a nucleon number of 35. Deduce the formula of the compound formed between elements D and E.
Solution:
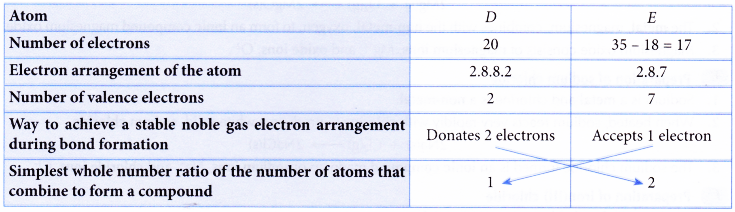
1 atom of element D combines with 2 atoms of element E to form a compound.
∴ Formula of the compound = DE2
2. Element X has a proton number of 13. An ion of element Y has 16 protons and 18 electrons. Deduce the formula of the compound formed between elements X and Y.
Solution:
Electron arrangement of atom X = 2.8.3
Element X has 3 valence electrons.
During bond formation, atom X loses 3 electrons to form a X3+ ion in order to achieve a stable octet electron arrangement.
Charge of a Y ion = Charge of 18 electrons + Charge of 16 protons
= (-18) + (+16)
= -2

2 atoms of element X combine with 3 atoms of element Y to form a compound.
∴ Formula of the compound = X2Y3
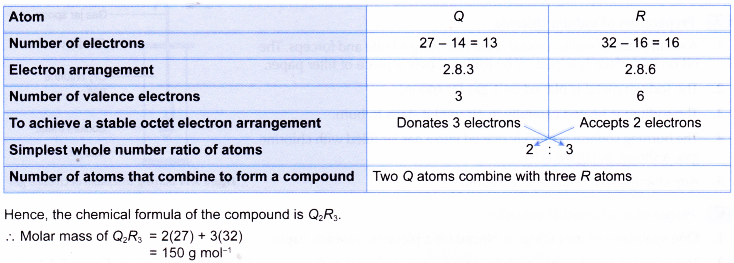
3. The following table shows the number of neutrons and the nucleon numbers of atoms of elements Q and R. Q and R are not the actual symbols of the elements.

Element Q reacts with element R to form a compound. What is the molar mass of the compound formed?
Solution: