How does a voltaic cell work?
- A voltaic cell is a device which converts chemical energy to electrical energy.
- The chemical reactions that take place inside the cell cause the flow of electrons and hence, electricity is produced.
- A simple voltaic cell is made by placing two different metals in contact with an electrolyte. The metals act as the electrodes for the voltaic cell. The electrodes are connected to a voltmeter, a galvanometer or an electric bulb using connecting wires.
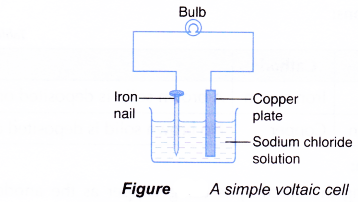
- The production of electricity in the cell will cause the bulb to light up, and make the needle of voltmeter or galvanometer to deflect.
- A voltaic cell is also called a galvanic cell.
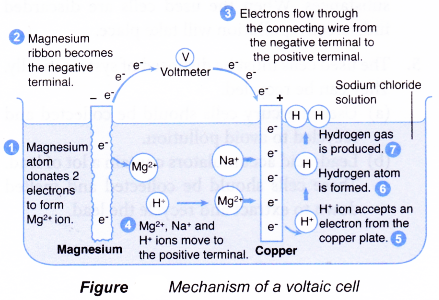
- The mechanism of a magnesium-copper simple cell with sodium chloride solution as the electrolyte is shown in Figure.
People also ask
- Why is an electrolyte able to conduct electricity while a Nonelectrolyte Cannot?
- Analysing the electrolysis of molten compounds
- Analysing the Electrolysis of Aqueous Solutions
- What does electrochemical series mean?
- How is electrolysis used in the industry?
- Electrolytic and Chemical Cells
- Oxidation and Reduction in Electrolytic Cells
- Oxidation and Reduction in Chemical Cells
How does the Daniell cell work?
- A zinc-copper cell or Daniell cell is another example of a voltaic cell.
- Figure shows the two structures of Daniell cells.
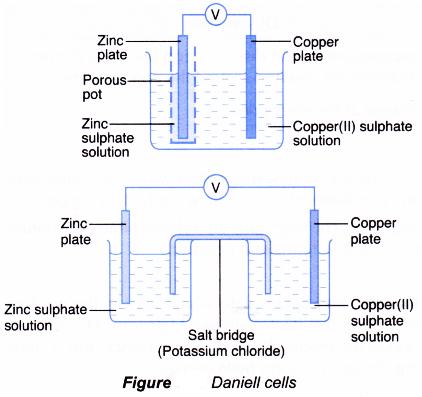
- In the cell, zinc and copper metals are used as the electrodes. Each of the metals is immersed into a solution of its ions.
The two electrolytes, zinc sulphate solution and copper(II) sulphate solution, are connected by a salt bridge or a porous pot. - Functions of a salt bridge or a porous pot are as follows:
(a) To prevent the two electrolytes from mixing.
(b) To allow the flow of the ions so that the electric circuit is completed. - A salt bridge is made from a piece of filter paper soaked in a saturated solution which does not react with the two electrolytes. Examples are saturated potassium nitrate solution, saturated sodium nitrate solution and dilute sulphuric acid. The salt bridge can also be a U-shaped tube that contains a saturated solution.
Reactions in a Daniell cell are as follows.
- Zinc which is higher than copper in the electrochemical series releases electrons more easily than copper. Each zinc atom donates two electrons to form a zinc ion. The zinc ions are released into the electrolyte. The zinc plate dissolves gradually.
At the negative terminal:
Zn(s) → Zn2+(aq) + 2e– - The electrons then flow through the connecting wire towards the copper plate which acts as the positive terminal. These electrons are accepted by the copper(II) ions to form copper metal. The copper plate becomes thicker gradually.
At the positive terminal:
Cu2+(aq) + 2e– → Cu(s)
The overall ionic equation:
Zn(s) + Cu2+(aq) → Zn2+(aq) + Cu(s) - The intensity of the blue colour of the copper(II) sulphate solution decreases as the concentration of the copper(II) ions decreases gradually.
- The flow of electrons from the zinc plate to the copper plate produces an electric current. The voltmeter shows a reading.
Voltaic Cell Experiment
Aim: To show the production of electricity from chemical reactions in a simple voltaic cell.
Problem statement: Can electricity be produced by chemical reactions in a simple voltaic cell?
Hypothesis: When two different metals are dipped into an electrolyte and connected by wires, electricity is produced.
Variables:
(a) Manipulated variable : Pairs of metals
(b) Responding variable : Voltmeter reading
(c) Controlled variable : Type of electrolyte
Operational definition: When an electric current is produced, the voltmeter needle deflects.
Materials: Magnesium ribbon, copper plate, 1 mol drrr sodium chloride solution and sandpaper.
Apparatus: 250 cm3 beaker, connecting wires with crocodile clips and voltmeter.
Procedure:
- A magnesium ribbon and a copper plate are cleaned with sandpaper.
- The two pieces of metals are connected using connecting wires as shown in Figure.
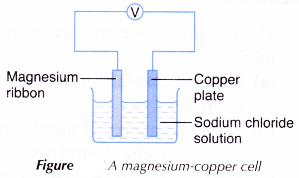
- The reading of the voltmeter is recorded. The changes at the electrodes are also recorded.
- Steps 1 to 3 are repeated using another copper plate to replace the magnesium ribbon.
Observations:
| Type of metal | Voltmeter reading (V) | Observation |
| Magnesium / copper | 2.7 | The magnesium ribbon dissolves and becomes thinner. Gas bubbles are released around the copper plate. |
| Copper / copper | 0.0 | No change at the electrodes. |
Discussion:
- When the magnesium ribbon and copper plate are used as the electrodes, magnesium which is higher than copper in the electrochemical series (or more electropositive) releases electrons more easily than copper. Each magnesium atom donates two electrons to form a Mg2+ ion. The Mg2+ ions are released into the sodium chloride solution while the electrons go onto the magnesium ribbon to make it negative.
At the negative terminal: Mg(s) → Mg2+(aq) + 2e–
The electrons then flow through the connecting wire towards the copper plate which acts as the positive terminal. These electrons can be accepted by the positive ions in the electrolyte like Mg2+ ions, H+ ions and Na+ions. The H+ ion is lower than the Mg2+ ion and Na+ ion in the electrochemical series. Hence, the H+ ions are selectively discharged at the copper plate by accepting electrons to form hydrogen gas.
At the positive terminal: 2H+(aq) + 2e– → H2(g)
The overall ionic equation: Mg(s) + 2H+(aq) → Mg2+(aq) + H2(g)
The flow of electrons from the magnesium ribbon to the copper plate results in the flow of electric current. - When the electrodes are made of copper metals, there is no potential difference between the copper electrodes. No flow of electrons occurs if both electrodes are made of the same metal.
Conclusion:
The chemical reactions in a simple voltaic cell produce electricity. No current will flow if both electrodes are made of the same metal. The hypothesis is accepted.
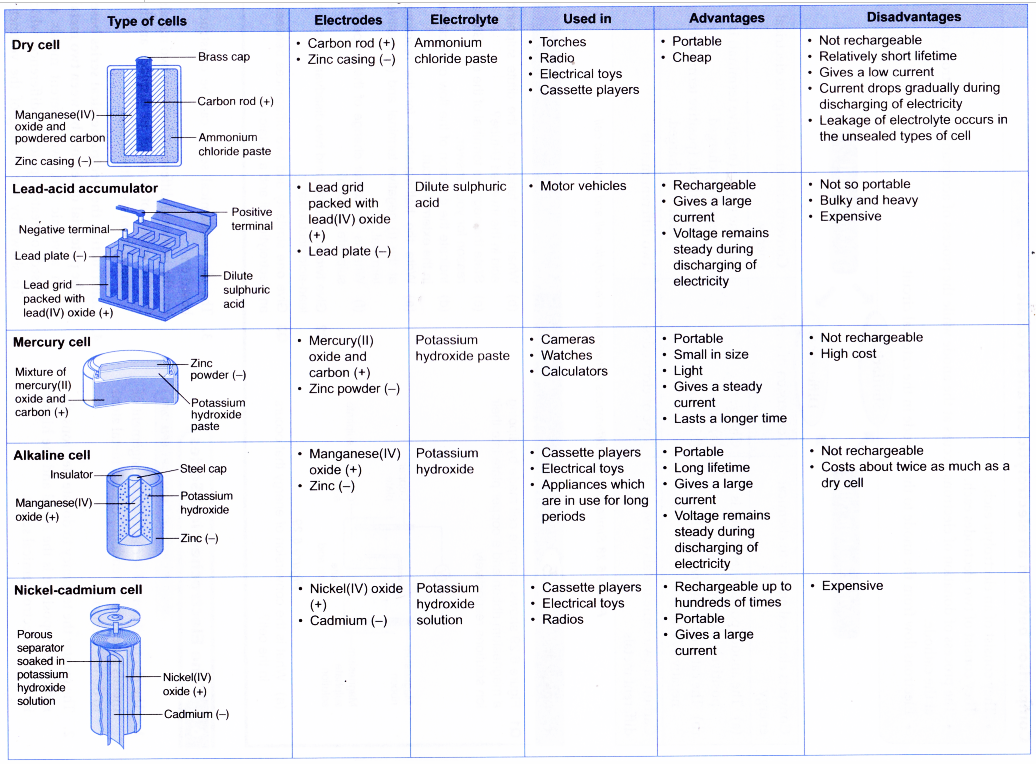
Advantages and disadvantages of various voltaic cells
- Various types of voltaic cells have been produced to suit our demand for the sources of portable electricity.
- The commonly used cells are the dry cell, lead- acid accumulator, mercury cell, alkaline cell and nickel-cadmium cell. The uses, advantages and disadvantages of these cells are summarised in Table.
- Other cells such as the lithium ion cell, nickel hydride cell and polymeric cell are now available.
- Most of the cells contain corrosive or poisonous substances. When the used cells are discarded indiscreetly, pollution will take place.
- The used cells must be disposed of systematically. They can be recycled.
(a) Used mercury cells should be collected and recycled to avoid pollution.
(b) Lead-acid accumulators contain a lot of lead. These cells should be collected and melted down to extract and recycle the lead.
Similarities and Differences Between Voltaic and Electrolytic Cells
Similarities:
- They consist of an electrolyte each.
- They consist of two electrodes each.
- The process of donation of electrons occurs at the anode while the process of acceptance of electrons occurs at the cathode.
- Electrons flow from the anode to the cathode in the external circuit.
Differences:
| Electrolytic cell | Differences | Voltaic cell |
| Converts electrical energy to chemical energy | Conversion of energy | Converts chemical energy to electrical energy |
| (a) The anode (positive terminal) is positively charged. (b) The cathode (negative terminal) is negatively charged. | Charge of (a) anode (b) cathode | (a) The anode (negative terminal) is negatively charged |
| Carbon or two same metals or two different metals | Type of electrodes | Two different metals |
Follow us on:
https://plus.google.com/+Veerendracbse
https://plus.google.com/+DurgaprasannaAmujuri
https://plus.google.com/+phanicbse
https://plus.google.com/+vinilachoudary
https://plus.google.com/+LearnCbseind
https://plus.google.com/+kajalsharmaLearnCBSE
https://plus.google.com/+sravyasharma
https://plus.google.com/+kajalsharmaLearnCBSE
https://plus.google.com/+cbseguesspapersfree
https://plus.google.com/+CharanSing
https://plus.google.com/+rajuheal
https://plus.google.com/+saralasharma
https://plus.google.com/+mastiaddaLearnCBSE
https://plus.google.com/+ChandraRamLearnCBSE
https://plus.google.com/+AmrutPandey
https://plus.google.com/+VenkataLakshmiLearnCBSE
https://plus.google.com/+nagaRajuLearnCBSE
https://plus.google.com/+SekharThe
https://plus.google.com/+TriveniMcc