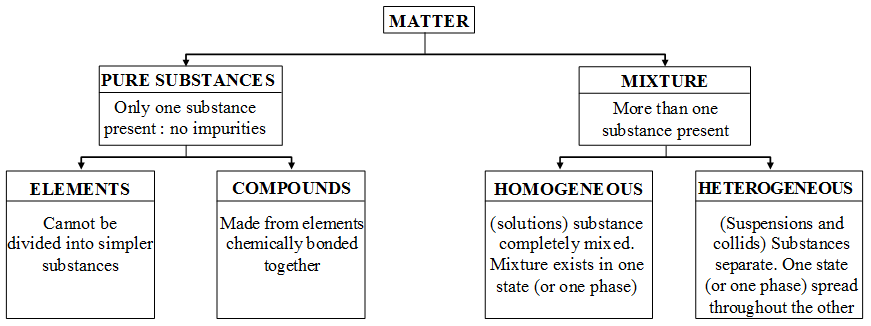
What are the Characteristics of Compounds
Compounds :
A compound is a substance made up of two or more elements chemically combined in a fixed proportion by mass. A compound is formed as a result of chemical reaction, between the constituent elements. The properties of compound are different from the properties of the elements from which it is formed.
 Ex. Water (H2O) is a compound made up of two elements, hydrogen and oxygen, chemically combined in a fixed proportion of 1 : 8 by mass
Ex. Water (H2O) is a compound made up of two elements, hydrogen and oxygen, chemically combined in a fixed proportion of 1 : 8 by mass
 Compounds can be further divided into three classes : acids, bases and salts, on the basis of their properties.
Compounds can be further divided into three classes : acids, bases and salts, on the basis of their properties.
Ex. Sulphuric acid is an acid, sodium hydroxide is a base whereas sodium sulphate is a salt.
Characteristics of a compound
- In a compound constituents are present in definite proportion by mass
- The properties of a compound are different from the properties of its constituents.
- The constituents of a compound cannot be separated by simple physical processes
- Formation of a compound is generally accompanied by evolution of energy in the form of heat or light.
- A compound has a fixed melting point and boiling point.
- A compound is always homogeneous in nature.

Read More: