Plus One Chemistry Chapter Wise Previous Questions Chapter 7 Equilibrium is part of Kerala Plus One Chemistry Chapter Wise Previous Year Questions and Answer . Here we have given Plus One Chemistry Previous Questions Chapter 7 Equilibrium.
Kerala Plus One Chemistry Chapter Wise Previous Questions Chapter 7 Equilibrium
Question 1.
The water solutions of the ionic compounds NaCI, CH3COONa and NH4CI show different pH. (March – 2009)
a) Identify the acidic, basic and neutral solutions among these.
b) Justify your answer.
Answer:
a) NaCI + H2O → NaOH + HCI, Hence neutral.
CH3COONa + H2O → CH3COOH + NaOH. Hence basic.
NH4CI + H2O → NH4OH + HCI. Hence acidic.
b) In aqueous solution, NaCI gives NaOH and HCI they are strong acid and base.
In aqueous solution, CH3COONa gives CH3COOH and NaOH. NaOH is strong.
In aqueous solution, NH4CI gives NH4OH and HCI. Among this HCI is strong acid.
Question 2.
When some sodium acetate is added to a solution of acetic acid, the concentration of unionized acetic acid increases. (March – 2010)
a) What is the phenomenon involved? Substantiate.
b) Consider the equilibrium,
![]()
The solubility of AgCI is 1.06 x 10-5molL-1 at 298K. Find out its KSp at this temperature.
c) What happens to the value of solubility and solubility product when HCI is passed through an AgCI solution?
Answer:
a) Common ion effect:- The dissociation of a weak acid can be decreased by adding a strong electrolyte having a common ion.
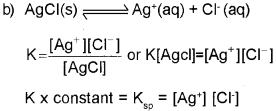
Question 3.
Lowry-Bronsted concept of acids and bases is based on the exchange of H+ during a reaction. (Say – 2010)
a) llustrate with an example of the conjugate acid-base pair.
b) Explain the Lewis concept of acids and bases.
c) According to Lewis theory classify the following into acids and bases:
1) H2O
2) NH3
3) AICI3
4) OH
Answer:
a) The base formed by the loss of proton from an acid is called the conjugate the base of the acid. Similarly the acid formed by gain of a proton by a base is called conjugate acid of the base.
The pairs of acids and bases which are formed from each other by the gain or loss of a proton are called conjugate acid-base pair.
Consider the equation.
![]()
This Cf and HCI constitute a conjugate acid-base pair. Similarly, H2O and H3O+ constitute another conjugate acid-base pair.
b) According to this concept an acid is defined as a substance which can accept a pair of electrons while a base is a substance which can donate a pair of electrons.
c) Acids → AICI3
Bases → H2O, NH3, OH’
Question 4.
Common ion effect is a phenomenon based on the Le-Chatelier’s principle. (March – 2011)
a) Illustrate the common ion effect using an example.
b) If the concentration of the hydrogen ion in a soft drink is 3 x 10-3 M, calculate its pH.
c) Identify the Lewis acids from the following :
i) OH–
ii) BCI3
iii) NH3
iv) H+
Answer:
a) The dissociation of a weak electrolyte can be suppressed by adding a strong electrolyte having a common ion.
eg : Dissociation of acetic acid can be suppressed by adding Sodium acetate to it.
b) pH= -log (H3O+)
pH = -log (3 x 10-3)
= -log 3 + 3 log 10
= -0.4771 + 3
= 3 – 0.4771
= 2.52
c) Lewis acid : BCI3, H+
Question 5.
The principal goal of chemical synthesis is to maximize the conversion of reactants into products. Le-Chatlier’s principle can be applied to achieve this goal. (Say – 2011)
a) State Le-Chatlier’s principle.
b) Predict the conditions to be applied to maximize the production of ammonia in the following reaction.
![]()
c) Comment on the effect of increase in pressure in the reaction
![]()
Answer:
a) The Le Chatelier’s principle states that a change in any of the factors that determine the equilibrium conditions of a system will cause the system to change in such a manner so as to reduce or to counteract the effect of the change,
b) In the manufacture of ammonia by Haber’s process ![]() the yield of ammonia can be increased by:
the yield of ammonia can be increased by:
- Increasing the concentration of N2 and H2.
- Removal of NH formed continuously by liquefaction from the reaction medium.
- Using an potimum temperature of 500°C and a pressure of 200 atm.
- By using spongy iron catalyst in presence of molybdenum as promoter or iron oxide with small amounts of K2O and Al2O3.
c) In the equilibrium ![]() number of moles increases in the forward reaction. On increasing pressure the equilibrium shifts in the direction in which there is decrease in the number of moles. Thus, increasing pressure shifts the equilibrium in the backward direction so that the decomposition of SO3 is suppressed.
number of moles increases in the forward reaction. On increasing pressure the equilibrium shifts in the direction in which there is decrease in the number of moles. Thus, increasing pressure shifts the equilibrium in the backward direction so that the decomposition of SO3 is suppressed.
Question 6.
Le Chatelier’s principle helps to explain the effect of change in conditions on equilibrium. (March – 2012)
Discuss the effect of pressure in the following equilibrium on the basis of Le Chatelier’s principle :
![]()
Answer:
![]()
The forward reaction involves a decrease in the number of molecules. Hence a high pressure will favour the formation of products.
Question 7.
The behavior of acids and bases can be explained using different concepts. (March – 2012)
a) Select the Lewis acid from the following : (NH3, OH–, BCI3, Cl–)
b) What are conjugate acid-base pairs? Illustrate using a suitable example.
OR
The pH of a salt solution depends on the hydrolysis of its ions.
a) Out of the following, which can produce an acidic solution in water? (CH3COONa, NH4CI, CH3COONH4, NaCI)
b) Explain the phenomenon of the common ion effect with a suitable example.
Answer:
a) Lewis acid : O\(\bar{H}\), C\(\bar{O}\)NH3
b) The pair of acid and base which is formed from each other by the gain or loss of a proton is called a conjugate acid-base pair.
Conjugate acid = Base + H+
Conjugate base = Acid – H+
![]()
OR
a) NH4CI
b) The ionisation of a weak electrolyte (weak acid or weak base) is suppressed by the addition of a salt which contains a common ion. This effect is called common ion effect. Eg: When weak electrolyte like acetic acid is present is solution.
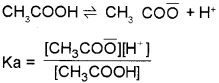
If another electrolyte, CH3COONa, which supplier CH3CO\(\bar{O}\) ion is added, the equilibrium will be disturbed. CH3COONa is a strong electrolyte and it completely ionises as CH3COONa
→ CH3CO\(\bar{O}\) + Na+. This leads to a considerable increase in concentration of CH3CO\(\bar{O}\) ion in the solution. This increase in concentration of CH3CO\(\bar{O}\) would shift the equilibrium considerably to the left (Le-Chatelier’s principle) ie CH3CO\(\bar{O}\) ions combine with H+ ions giving unionised CH3COOH. Thus degree of ionisation of acetic acid is suppressed by the addition of CH3COONa.
Question 8.
a) During the classroom discussion one of your friends argues that equillibrium constant is not altered with change in temperature. What is your view towards this argument? Justify. (Say – 2012)
b) Dissociation of CaCO3 in a closed vessel is given as
![]()
i) Write an expression for Kc.
ii) Explain the effect of increase in pressure on the above reaction. Name the principle behind this.
Answer:
a) Equillibrium constant varies with temperatures. Its variation with temperature can be described by Arrhenius equation.
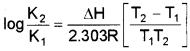
where
ΔH → Enthalpy change k1, & k2 → Eqbm constant at temperature T1 & T3. R → Universal gas constant.
This equation is known as Van’t Hoff equation.
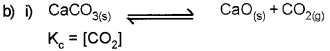
ii) Pressure increases – Equilibrium shift towards lesser no. of gaseous moles.
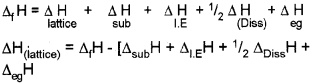
Name of the principle behind this is Le- Chatelier’s principle.
Question 9.
Equilibrium is possible only in a closed system at a given temperature. (March – 2013)
a) Write the expression for equilibrium constant Kc for the reaction.
![]()
b) What happens to the value of the equilibrium constant (K‘c) when the above reaction is reversed?
Answer:
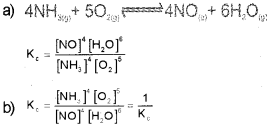
Question 10.
Weak acids are partially ionized in aqueous solutions.
a) The ionization constants of some acids are given below:
Acid – Ionization constant (Ka)
Formic acid (HCOOH) – 1.8 x 10-4
Hypocholorous acid (HCIO) – 3.0 x 10-8
Nitrous acid (HNO2) – 4.5 x 10-4
Hydrocyanic acid (HCN) – 4.9 x 10-10
Arrange the above acids in the increasing order of their acid strength.
b) Calculate the pH of 0.01 M acetic acid solution with the degree of ionization 0.045.
OR
Salts can be classified into different categories on the basis of their solubility.
a) Identify the solubility range of sparingly soluble salts from the following: (Between 0.01 M and 0.1 M, less than 0.01 M, greater than 0.1M, greater than 0.1 M).
b) Calculate the solubility (S) of CaSO4 at 298 K if its solubility product constant (Ksp) at this temperature is 9 x 10-6
Answer:
a) Ionization constant (ka) of acid increase Acidity increases.
∴ Increasing order of acid strength
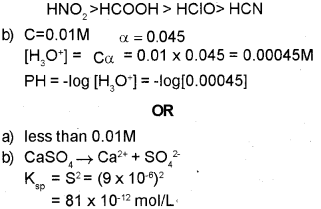
Question 11.
a) What is the conjugate acid-base pair? Illustrate with an example. (Say – 2013)
b) Define the pH scale. The pH of a soft drink is 2.42. Give the nature of the solution.
c) An aqueous solution of CuSO4 is acidic while that of Na2SO4 is neutral. Explain.
Answer:
a) The acid-base pairthat differs by a proton is called conjugate acid-base pair.
e.g. in the ionisation of hydrochloric in water, HCI(aq) acts as acid by donating a proton to H2O molecule which acts as a base.
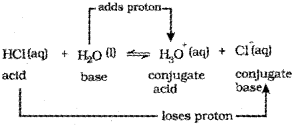
In the above equation, water acts as a base be-cause it accepts the proton. The species H3O+ is produced when water accepts a proton from HCI. Therefore, Cl– is a conjugate base of HCI and HCI is the conjugate acid of base Cl. Similarly, H2O is a conjugate base of an acid H3O+ and H3O– is a conjugate acid of base H2O.
b) In pH scale, pH of a solution is defined as the negative logarithm to base 10 of the activity (aH+) of hydrogen ion.
pH = -log[H+]
pH 2.42 is acidic because acidic solution has pH less than 7
c) CuSO4, being a salt of a strong acid H2SO4 and a weak base Cu(OH)2 gets completely ionised in aqueous solution.
![]()
CU2+(aq) thus formed undergoes hydrolysis in water to give Cu(OH)2 and H+ ions.
![]()
Na2SO4 is a salt formed from a strong base NaOH and a strong acid H2SO4. Hence, it has no hydrolysis. Therefore, its aqueous solution is neutral.
Question 12.
a) Write an equation for equilibrium constant in terms of concentration (Kc) for the equilibrium reaction given below. (March – 2014)
![]()
b) What are buffer solutions? Give an example of fora buffer solution.
c) The concentration of H+ ion in a sample of soft drink is 3.8 x 10-3 M. Determine its pH.
Answer:
![]()
b) Solutions which resist change in pH on dilution or with the addition of small amounts of acid or alkali are called buffer solutions.
e.g., a mixture of CH3COOH and CH3COONa (acid buffer), a mixture of NH4CI and NH4OH (basic buffer)
c) pH = -log[H3O+]
pH = -log[3.8 x 10-3] = 2.42
Question 13.
Le-Chatelier’s principle makes a qualitative prediction about the change in conditions on equilibrium. (August – 2014)
a) State Le-Chatelier’s principle.
![]()
What is the effect of pressure on the above equilibrium?
c) The species HCO3 and HSO4 can act both as Bronsted acids and bases. Write the corresponding conjugate acid and conjugate base of the above species.
Answer:
a) It states that a change in any one of the factors that determine the equilibrium conditions of a system will cause the system to change in such a manner so as to reduce or to counteract the effect of the change.
b) Pressure change has no effect in this equilibrium since the total number of moles of gaseous reactants is equal to the total number of gaseous products.
c) HCO3- Conjugate acid – H2CO3, Conjugate base – CO32+
HSO4– – Conjugate acid-H2SO4, Conjugate base – SO42-
Question 14.
a) i) Give the Arrhenius concept about acids and bases. (March – 2015)
ii) Give one example each for Arrhenius acid and base.
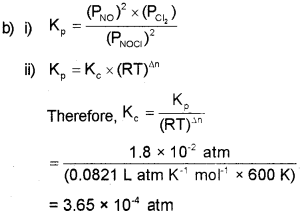
b) i) Write the expression for equilibrium constant Kp for the following equilibrium.
![]()
ii) Find the value of Kc for above equilibrium if the value of Kp is 1.8 x 10-2 atm at 600 K. R = 0.0821 L atm K-1 mol-1.
Answer:
a) i) According to Arrhenius theory, acids are substances that dissociates in water to give hydrogen ions, H+(aq) and bases are substances that produce hydroxyl ions, OH (aq).
ii) Example for Arrhenius acid – HCI(aq) H2SO4(aq), HNO3(aq) -anyone.
Example for Arrhenius base – NAOH(aq), KOH(aq) – any one.
Question 15.
Equilibrium constant helps in predicting the direction helps in predicting the direction in which a given reaction will proceed at any stage. (Say – 2015)
a) In which one of the following conditions a chemical reaction proceeds in the forward direction?
i) Qc < Kc
ii) Qc > Kc
iii) Qc = 1/Kc
iv) Qc = -Kc
b) Write whether the following statement is true or false.
“High value of equilibrium constant suggests high concentration of the reactants in the equilibrium mixture”.
c) State the Le-Chatelier’s principle.
![]()
Applying this principle, explain the effect of pressure in the following equilibrium.
Answer:
a) i) Qc < Kc
b) False
c) The Le Chatelier’s principle states that a change in any of the factors tat determine the equilibrium conditions of a system will cause the system to change in such a manner so as to reduce or to counteract the effect of the change.
On increasing pressure the rate of forward reaction increases as it is associated with decrease in number of moles.
On decreasing pressure the rate of backward reaction increases as it is associated with increase in number of moles.
Question 16.
a) Write the expression for equilibrium constant Ke for the following equilibrium. (March – 2016)
b) The solubility product of Al (OH)3 is 1 x 10-36. Calculate the solubility of Al (OH)3.
OR
a) Explain the concept of Lewis acids and Lewis bases with suitable examples.
b) Write the Henderson-Hasselbalch equation for an acidic buffer, Calculate the pH of an acidic buffer containing 0.1 M CH3 COOH and 0.5 M CH3COONa [Ka for CH3COOH is 1.8 x 10-6]
Answer:
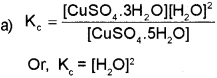
Since, the molar concentration of pure solids is constant and is taken as unity,
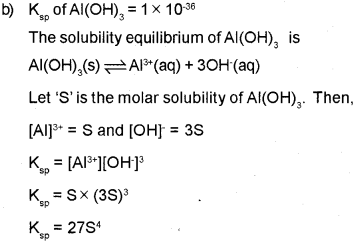

OR
a) According to Lewis concept acid is a species which accepts electron pair and base is a species which donates an electron pair. Example for Lewis acids – BF3,
AICI3, H–, Co3+ etc.
Examples for Lewis bases – H2O, NH3, OH’ etc.
b) The Henderson – Hasselbalch equation for an acidic buffer is
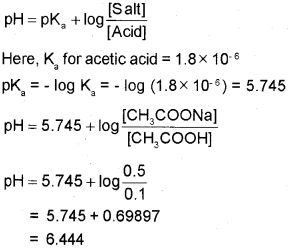
Question 17.
a) The solubility product of salt is related to its solubility. (Say – 2016)
i) Give the relation between solubility product and solubility of BaS04.
ii) The solubility product of BaS04 is 1.2×10’10 at 298 K. Calculate the solubility of BaS04 at 298 K.
b) Differentiate between homogeneous and heterogeneous equilibria.
Answer:
a) i) The equilibrium between the undissolved solid and the ions in a saturated solution of BaSO4, can be represented by the equation:
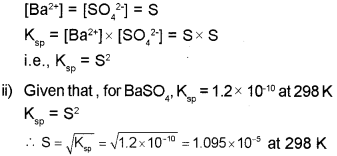
b) Homogeneous equilibria: Equilibria in which all the reactants and products are in the same phase.
![]()
Here, all the reactants and products are in the gaseous phase.
Heterogeneous equilibria: Equilibria in a system having more than one phase i.e., reactants and products are in a different phase, ![]()
Here, there is a solid phase and a liquid phase.
Question 18.
a) Classify the following solutions into acidic, basic and neutral NaCI, NH4NO3, NaCN, NaNO2. (March – 2017)
b) PH of blood remains constant in spite of the variety of goods and spices we eat. Give a reason.
c) The solubility of Mg(OH)2 at 298k is 1.5 x 10-4. Calculate the solubility product.
Answer:
a) Acidic-NH4NO3
Basic – NaCN, NaNO2
Neutral – NaCI
b) This is due to the presence of a buffer system in blood. The carbonate/bicarbonate (H2CO3/HCO3) buffer system helps to maintain pH of blood between 7.26 to 7.42
c) Given that, Solubility of Mg(OH)2, S = 1.5 x 10-4 The equilibrium between the undissolved solid an ions in a saturated solution of Mg(OH)2 can be represented as,
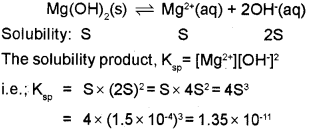
We hope the Kerala Plus One Chemistry Chapter Wise Previous Questions Chapter 7 Equilibrium help you. If you have any query regarding Kerala Plus One Chemistry Chapter Wise Previous Questions Chapter 7 Equilibrium, drop a comment below and we will get back to you at the earliest.