Plus One Chemistry Chapter Wise Previous Questions Chapter 6 Thermodynamics is part of Kerala Plus One Chemistry Chapter Wise Previous Year Questions and Answer . Here we have given Plus One Chemistry Previous Questions Chapter 6 Thermodynamics.
Kerala Plus One Chemistry Chapter Wise Previous Questions Chapter 6 Thermodynamics
Question 1.
a) State Hess’s Law of constant heat summation. (March – 2009)
b) The equilibrium constant for a reaction is 5. What will be the value of ΔG°.
Given that R = 8.314JK-1 mol-1, T = 300K.
R = 8.314 JK-1 mol-1
T = 300K
Answer:
a) Hess’s law : Enthalpy change in a chemical reaction is same whether it takes place in one step or more than one step,
b) ΔG = -2.303RT logK
ΔG = -2.303 x 8.314 x 300 x log5
= -2.30 x 8.314 x 300 x 0.6989
= -4014.581 J/mol
Question 2.
A system in thermodynamics refers to that part of the universe in which observation are made. (March – 2010)
a) What do you mean by an isolated system? Given an example.
b) Distinguish between intensive and extensive properties. Give two examples for each.
Answer:
a) A system which can neither exchange matter nor energy with the surrounding. It is called as isolated system.
Eg: Thermo flask
b) Extensive property : The properties of the system which depend upon the quantity of the matter contained in the system.
Eg: mass, volume.
Intensive property : The properties of the system whose values are independent of the quantity of substance present in the system.
Eg: Temperature, pressure.
Question 3.
Lattice enthalpy of an ionic salt is a factor that determine its stability. (Say – 2010)
a) Define the lattice enthalpy.
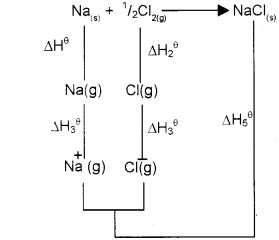
b) Draw the Born-Haber cycle for the calculation of lattice enthalpy of the ionic crystal NaCI.
Answer:
a) The lattice enthalpy of an ionic solid is defined as the energy required to completely separate one mole of the ionic compound into gaseous constituent ions.
b) Na(s) + 1/2CI2(g) → NaCI(s)
Question 4.
The spontaneity of a process is expressed in terms of a change in Gibbs energy. (March – 2011)
a) What is meant by a change in Gibbs energy of a system?
b) How is it related to the enthalpy and entropy of a system?
c) How is it useful in predicting the feasibility of a process?
Answer:
a) Free energy is the energy available to a system that can be converted into useful work.
b) ΔG = ΔH – TΔS
c) For a spontaneous process, ΔG = –ve
For Non-Spontaneous process ΔG = +ve
If the system is at equilabrium ΔG = O
Question 5.
A spontaneous process is an irreversible process and may only by reserved by some external agency. (Say – 2012)
a) Decrease in enthalpy is the only criterion for spontaneity. Do you agree? Why?
b) Calculate the work done for the reversible iso thermal expansion of 1 mole of an ideal gas at 27°C, from a volume of 10 dm3 to a volume of 20dm3.
Answer:
a) No. Decrease in enthalpy (ΔH = —ve) is not the only criterion for spontaneity. There are many endothermic processes which are also spontaneous like the evaporation of water placed in an open vessel. A process takes place spontaneously not only in the direction in which enthalpy of the system is reduced but also in the direction in which disorder or randomness increases (ΔS = +ve ). Thus, a resultant of the tendencies to decrease enthalpy and increase entropy determines the spontaneity of a process.

Question 6.
Thermodynamics deals with energy changes of macroscopic systems. (March – 2012)
a) Consider a chemical reaction taking place in a dosed insulated vessel. To which type of ther modynamic system does it belong?
b) State the first law of thermodynamics.
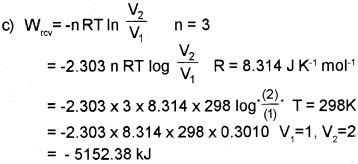
c) 3 moI of an ideal gas at 1.5 atm and 25°C expands isothermally in a reversible mannerto twice its original volume against an external pressure of 1 atm. Calculate the work done. [R = 8.314 JK-1 mol-1]
Answer:
a) Isolated system
b) Ist law of thermodynamics states that ‘the energy of an isolated system is Constant’.
Question 7.
a) i) Construct an enthalpy diagram for the determination of lattice enthalpy of sodium chloride. (Say – 2012)
ii) Enthalpy and entropy changes of a reaction are40.63kJ/mol and 108.8 Jk-1 mol-1. Predict the feasibility of the reaction atr 27°C.
OR
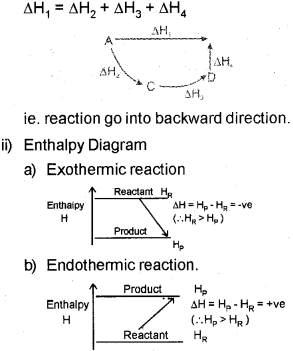
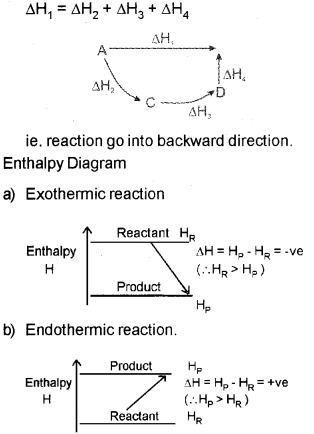
b) i) Explain the Hess’s law of constant heat summation, with an example.
ii) Draw the enthalpy diagram for an exothermic and endothermic reaction.
Answer:
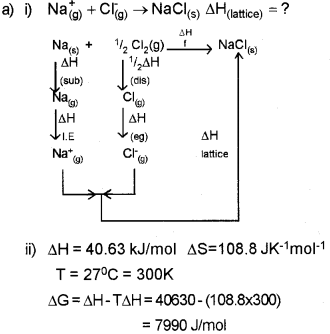
Since ΔG is +ve, the reaction is not feasible at 27°C.
OR
b) i) Hes&s law states that the enthalpy change during a process is the same whether ¡t takes place in one step or in several steps.
Then according to Hess’s law
Question 8.
Most of the naturally occurring processes are spontaneous. (March – 2013)
a) Give the criteria for spontaneity of a process in terms of free energy change (ΔG)
b) Exothermic reactions associated with a decrease in entropy are spontaneous at lower temperatures. Justify on the basis of Gibbs equation.
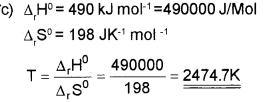
c) Find the temperature above which the reaction
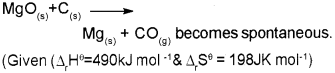
Answer:
a) Free energy change (ΔG) is negative for a spontaneous process.
b) Far a Process to be spontaneous ΔG should be -ve. But ΔG = ΔH -T ΔS ………….. (1)
For Exothermic reaction ΔH =’ve
and decrease in entropy ΔS = -ve.
Substitute the value of ΔH and ΔS in (1) ΔG become -ve only when ΔH > T ΔS. Such reactions are spontaneous only at low temperatures.
At 2474.7K and above this temperature the reaction become spontaneous.
Question 9.
a) The enthalpy of combustion of CH4(g), C(Graphite) and H2(g) at 298 K are -890.3 kJ mor-1, -393.5 kJ mor-1 and -285.8 kJ moM respectively. Calculate the enthalpy of formation of CH4(g). (Say – 2013)
b) Match the following:

Answer:
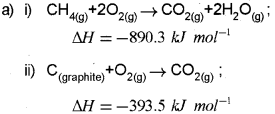
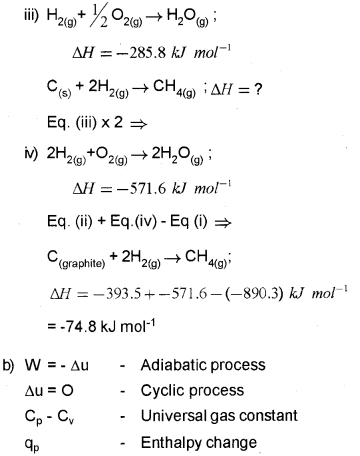
Question 10.
a) For the oxidation of iron 4 Fe(s) + 3O2(g) → 2 Fe2O3(s), entropy change is -549.4 JK-1 mol-1 at 298 K. Inspite of the negative entropy change of this reaction, why is the reaction spontaneous? (ΔH°r for the reaction is -1648 x 10 Jmol-1). (March – 2014)
b) Write the dfference between extensive and intensive properties. Give one example of each.
Answer:
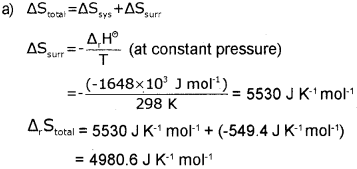
b) Extensive properties – properties whose values depend on the quantity or size of matter present in the system.
e.g., Mass, Volume. Internal energy. Enthalpy, Heat capacity etc.
Intensive properties- properties which do not depend on the quantity or size of matter present in the system.
e.g., Temperature, Density, Pressure etc.
Question 11.
a) ΔG gives a criteria for spontaneity of reactions at a constant pressure and temperature. How is ΔG helpful in predicting the spontaneity of the reaction? (August – 2014)
b) State and explain Hess’s law of constant heat summation.
Answer:
a) If ΔG is negative (<O), the proces is spontaneous. If ΔG is positîve (>0), the proces is non-spontaneous.
b) Hess’s law states that the enthalpy change during a process is the same whether it takes place in one step or in several steps.
Then according to Hess’s law
Question 12.
a) Classify the following into intensive and extensive properties. (March – 2015)
i) Inernal energy
ii) Density
ii) Heat Capacity
iv) Temperature
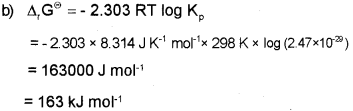
b) Calculate the standard free energy change (ΔG°) for the conversion of oxygen to ozone 3/2 O2(g) → O3(g) at 298 K if the equilibrium constant for the conversion is 2.47 x 10-29. (Given R = 8.314 JK-1 mol-1)
Answer:
a) Intensive properties –
ii) Density and
iv) Temperature
Extensive properties-
i) Internal energy
iii) Heat capacity
Question 13.
Expansion of a gas in vacuum is called free expansion. (Say – 2015)
a) Which one of the following represents free expansion of an ideal gas under adiabatic conditions?
i) q = 0, ΔT ≠ 0, w = 0
ii) q ≠ 0, ΔT = 0, w= 0
iii) q = 0, ΔT = 0, w = 0
iv) q = 0, ΔT < 0, w = 0
b) The enthalpy change for the reaction.
![]()
Answer:
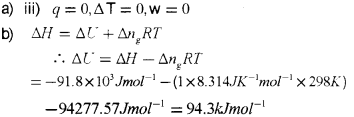
Question 14.
The enthalpy change in a process is the same, whether the process is carried out in a single step or inseveral steps. (March – 2016)
a) Identify the law stated here.
b) Calculate the enthalpy of formation of CH4 from the following data:
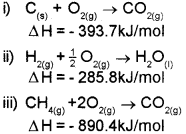
Answer:
a) Hess’s law
b) The required equation is
![]()
The given data are:

Question 15.
a) Which of the following is a process taking place with increase in entropy? (Say – 2016)
i) Freezing of water
ii) Condensation of steam
iii) Cooling of a liquid
iv) Dissolution of a solute
b) State and illustrate Hess’s law.
Answer:
a) iv) Dissolution of a solute
b) Hess’s law states that if a reaction takes place in several steps then its standard reaction enthalpy is the sum of the standard enthalpies of the intermediate reactions into which the overall reaction may be divided at the same temperature. Illustration:
Consider the formation of CO2 from carbon and oxygen. There are two ways by which the change can be brought about.
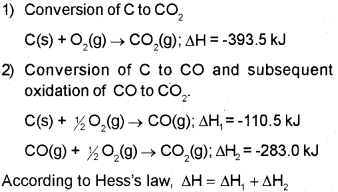
In general, if enthalpy of an overall reaction A → B along one route is Δ1H and Δ1H1, Δ2H2, Δ3H3 …. representing enthalpies of reactions leading to same product, B along another route, then according to Hess’s law,
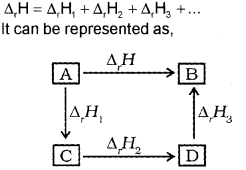
Question 16.
a) Some macroscopic properties are given below. (March – 2017)
Help Reena to classify then into two groups under suitable titles [Heat capacity, Entropy, Refractive index, Surface tension] (2)
b) For the reaction
2A(g) + B(g) → 2D(g)
AU° = -10.5kJ/mol
ΔS° = -44.1J/k/mol at 298k.
Calculate AG°forthe reaction
Answer:
a) Extensive properties: Heat capacity, Entropy Intensive properties: Refractive index, Surface tension

We hope the Kerala Plus One Chemistry Chapter Wise Previous Questions Chapter 6 Thermodynamics help you. If you have any query regarding Kerala Plus One Chemistry Chapter Wise Previous Questions Chapter 6 Thermodynamics, drop a comment below and we will get back to you at the earliest.