Plus One Chemistry Chapter Wise Previous Questions Chapter 11 The p-Block Elements is part of Kerala Plus One Chemistry Chapter Wise Previous Year Questions and Answer . Here we have given Plus One Chemistry Previous Questions Chapter 11 The p-Block Elements.
Kerala Plus One Chemistry Chapter Wise Previous Questions Chapter 11 The p-Block Elements
Question 1.
A compound is formed between oxygen and flurine. Do you know whether it is oxygen fluride or flurine oxide? Explain. (February – 2008)
NO and HNO3 are two compounds of N. In which of them is N2 more oxidized?
Answer:
Oxygen fluride
In HNO3, Nitrogen is more oxidised.
Question 2.
a) Briefly describe the structure of diborane. (March – 2009)
b) What is inorganic benzene?
Why is it called?
Answer:
a) Diborane has an unusual structure.
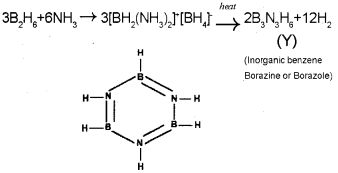
b) B3N3H6 -Borazine.
It is commonly known as inorganic benzene due to its structural similarity with benzene.
Question 3.
Boron, Aluminium, Gallium, Indium and Thallium belong to group 13 of the periodic table of elements. (March – 2010)
a) Howcan you explain a higher stability of BCI3 as compared to TICI3?
b) While Aluminium can be form the ion [AIF6]3-, Boron is unable to form [BF6]3- ion. Explain.
c) State whether the compound BCI3 is acidic or basic.
d) Write the hybridization state of B in BF3 and BH4
Answer:
a) Due to small size of Boron
b) Due to absence of d-orbitals in Boron
c) Acidic
d) BF3 → sp2
BH4 → sp3
i) Diamond, Graphite, Fullerenes
ii) Graphite
Question 4.
Match the following. (Say – 2010)
| A | B | C |
| 1) Inorganic Benzene | a) Allotrope | i) Aluminium silicates |
| 2) Glass like beads | b) Borax | ii) Carbon |
| 3) Fullerene | c) Borazine | iii) B2H6 |
| 4) Zeolite | d) Dry Ice | iv) B3N3H6 |
| e) Softening of hard water | v) Na2[B4O5(OH)4.8H20 |
Answer:
| A | B | C |
| 1) Inorganic Benzene | Borazine | B3N3H6 |
| 2) Glass like beads | Borax | Na2[B405(0H)4.8H20 |
| 3) Fullerene | Allotrope | Carbon |
| 4) Zeolite | Softening of hard water | Aluminium silicates |
Question 5.
Two important oxides of carbon are carbon monoxide and carbon dioxide. (March – 2011)
a) Why is CO called a poisonous gas?
b) What are producer gas and water gas? Mention their uses.
Answer:
a) Carbon monoxide reacts with haemoglobin to form carboxy haemoglobin.
b) Producergas is a mixture of CO and N2 and water gas is a mixture of CO and H2. They are used as fuels.
Question 6.
a) Some elements can exit in different Crystaline forms and are called allotropes (Say – 2011)
i) What are the three important allotropic forms of carbon?
ii) Which allotropic form of carbon is used as a dry lubricant in machines running at high temperature.
b) When sodium borohydride (NaBH4) is treated with iodine (l2), two gaseous products were obtained. One is hydrogen and the other is a highly toxic gas X, which catches fire upon exposure to air. When the gas X is heated with ammonia for a long time, a compound Y of ring structure is obtained. Identify X and Y. (Name and molecular formula are expected)
Answer:
a) i) Diamond, Graphite, Fullerenes
ii) Graphite
![]()
‘X’ is diborane (B2H6). It is a highly toxic gas and catches fire spontaneously upon exposure to air.
B2H6 + 3O2 → B2O3 + 3H2O; ΔCH0 -1976 kJ mol-1
Reaction of ammonia with diborane gives initially B2H6.2NH3 which is formulated as [B2H6(NH3)2]+ [BH4]-. Further heating gives borazine, B3N3H6 known as ‘inorganic benzene’ in view of its ring structure with alternate BH and NH groups.
Question 7.
Borax, orthoboric acid and diborane are some useful compounds of boron. (March – 2012)
a) Write the chemical formula of borax.
b) Boric acid is not a protonic acid but acts as a Lewis acid. Justify.
c) Explain the structure of diborane using a diagram.
Answer:
a) Borax – Na2B4O7.10H2O
b) Boric acid is a monobasic acid. It is not a protonic acid. It acts as a Lewis acid. It is not able to release H+ ions of its own. It receive OH ions from water molecule to complete its octet and in turn release H+ ions
B(OH)3 + H – OH → [B(OH)4]+ + H+
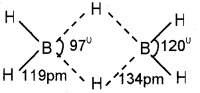
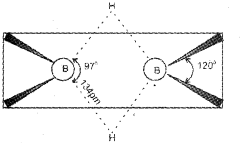
c) In diborane, all the six hydrogen atom are not similar. Four are terminal hydrogen atoms and two are bridged. The boron atoms and four-terminal Hydrogen atom lie in one plane. The bridged hydrogen atom lie above and below this plane. The terminal B-H bonds are regular bonds. But the bridged B-H bonds are three centre two-electron (3c -2e) bonds or banana bond.
Question 8.
a) Diborane is an electron-deficient compound. (Say – 2012)
i) Name the special bonds that present in Diborane.
ii) How will you convert Diborane into inorganic benzene?
b) What are silicones? Write its General formula.
Answer:
b) Silicones are polymeric organosilicon compound having C-Si and Si-O-Si bonds or R2SiO units General formula – R2(SiO)n
R → methyl or phenyl group.
Question 9.
The group 14 elements have four electrons in the outermost shell. (March – 2013)
a) SiCI4 can be easily hydrolyzed by water while CCI4 cannot be hydrolyzed.
b) How are fullerenes prepared?
c) Distinguish between silicones and silicates.
Answer:
a) SiCI4 can be easily hydrolysed by water because due to the availability of empty d-or bitals in thier ‘Si’- atom. But CCI4 cannot be hydrolysed due to absence of ‘d’-oritals in their ‘c’ -atom.
b) Fullerenes Eg: C60 Preparation Twenty hexagonal (6-membered) rings and twelve pentagonal (5-membered) rings of SP2 hybridised carbon atoms fused in to each other. Each Carbon atom forms 3 α bonds with other 3 Carbon atom. The remaining electron at each carbon is delocalised in molecular orbitals.
c) Silicones are ploymeric organo silicon compounds having C-Si and Si-O-Si bonds. They have general formula (R2SiO)n.
Where R = Methyl or phenyl group.
Silicate are minerals of Silicon and occurs in earth’s Crust in the form of numerous minerals and clays. The basic building unit of all silicates in tetrchedral SiO44- ion.
Question 10.
a) i) Boric acid (H3BO3) is considered as a weak acid Why? (Say – 2013)
ii) Carbon monoxide is highly poisonous. Why?
b) What are zeolites? What is its use?
a) i) Boric acid is a a weak monobasic acid. It is not a protic acid but acts as a Lewis acid by accepting electrons from a hydroxyl ion.
B(OH)3 + 2HOH → [B(OH)4]– + H3O+
ii) The highly poisonous nature of CO arises because of its ability to form a complex with haemoglobin, which is about 300 times more stable than the oxygen-haemoglobin complex. This prevents haemoglobin in the red blood corpuscles from carrying oxygen round the body and ultimately resulting in death.
b) Zeolites are hydrated aluminosilicates of metals. In zeolites some of the silicon atoms in the three-dimensional network of silicon dioxide are replaced by aluminium atoms. They have honey comb like structure. They are widely used as catalysts in petrochemical industries for cracking of hydrocarbons and isomerisation. Hydrated zeolites are used as ion exchangers in softening of hard water.
Question 11.
a) What is dry ice? (March – 2013)
b) Why does BF3 behave as a Lewis acid?
c) Carbon forms millions of compounds due to its self-linking property to form long chains and big rings.
i) Name the above property of carbon.
ii) Give the reason for the above property of carbon.
Answer:
a) Solid carbon dioxide
b) In BF3, there are only six electrons around the central atom B. Thus, BF3 is electron-deficient and has a tendency to accept a pair of electrons to achieve stable electronic configuration. Hence, it behaves as a Lewis acid.
c) i) Catenation
ii) The property of catenation depends upon the strength of atom-atom bond. Thegreaterthe strength of the atom-atom bond, greater is the extent of catenation. Jt is most extensive in the case of carbon because C-C bond is very strong.
Question 12.
Give reasons for the following: (August – 2014)
a) CO2 is a gas whereas SiO2 is a solid.
b) CCI4 cannot be hydrolyzed but SiCI4 can be hydrolysed.
c) Borax bead test can be used to identify metaborates in the laboratory.
d) Graphite is used as a lubricant in machines.
Answer:
a) Due to the presence of double bond between C and O in CO2 they exist as discrete molecules. But in SiO2 there is a three-dimensional network structure in which each silicon atom is covalently bonded in a tetrahedral manner to four oxygen atoms. Each oxygen atom, in turn, is covalently bonded to other silicon atoms. The entire crystal may be considered as a giant molecule in which eight-membered rings are formed with alternate Si and O atoms. Hence, SiO2 is a solid with high melting point.
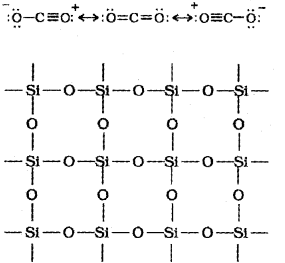
b) The maximum covalence of C is 4 and it has no d-orbitals to accept the lone pair of electrons from water molecules. But Si can expand its covalence beyond 4 and has vacant d-orbitals. Hence SiCI4 undergoes hydrolysis by initially accepting lone pair of electrons from water molecule in d- orbitals of Si, finally leading to the formation of Si(OH)4.
c) The metaborates of many transition metals have characteristic colours and, therefore, borax bead test can be used to identify them in the laboratory.
d) Graphite has a layered structure. It cleaves easily between the layers and, therefore, it is very soft and slippery. For this reason graphite is used as a dry lubricant in machines running at high temperatures, where oil cannot be used as a lubricant.
Question 13.
a) Thermodynamically, the most stable allotrope of carbon is ……… (March – 2015)
b) Carbon is the first member of group 14 in the periodic table.
i) Why does carbon differ from the rest of the members of its group?
ii) Write any two anomalous properties of carbon.
Answer:
a) Graphite
b) i) Carbon is the first member of group 14. Like first members of other groups it differs from the rest of the members of its group due to its smaller size, higher electronegativity, higher ionisayion enthalpy and unavailability of ‘d’ orbitals.
ii)
1) Maximum covalency is four since in carbon, only ‘s’ and ‘p’ orbitals are available for bonding and, therefore, it can accomodate only four pairs of electrons around it.
2) Carbon has unique ability to form pπ – pπ multiple bonds with itself &with other at-oms of small size and high electronegativity.
3) Catenation – Carbon atoms have the tendency to link with one another through covalent bonds to form chains and rings.
Question 14.
Orthoboric acid is an important compound of boron: Prepare a short note on orthoboric acid highlighting the following aspects: (Say – 2015)
- Method of preparation
- Acidic nature
- Action of heat
- Structure
Answer:
Method of preparation: It is prepared by acidifying an aqueous solution of borax.
Na2B4O7 + 2HCI + 5H2O → 2NaCI + 4B(OH)2
Acidic nature: It acts as a weak monobasic Lewis acid by accepting electrons from a hydroxyl ion.
B(0H)3 + 2HOH → [B(OH)4] + H3O+
Action of heat: On heating above 370 K it forms metaboric acid (HBOz) which on further heating yields boric oxide (B2O3).
![]()
Structure: It has a layer structure in which planar BO3 units are joined by hydrogen bonds.
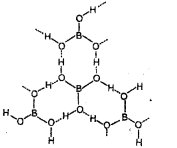
Question 15.
Carbon has many allotropes. (March – 2016)
a) Write the name of any two allotropic forms of carbon.
b) Briefly explain the structure of any one of the above-mentioned allotrope.
C) CCI4 does not undergo hydrolysis. Give a reason.
OR
When BF3 is treated with LiH at 450 K, a hydride of carbon is formed.
a) Identify the hydride of boron formed in the above reaction.
b) Briefly explain the structure of the above-mentioned hydride.
c) BoroncompoundsbehaveasLewisaciclsWhy?
Answer:
a) Diamond, graphite, fullerene (any two)
b) Structure of diamond – it has a crystalline lattice. Each carbon atom undergoes sp3 hybridisation and is linked to four other carbon atoms by using hybridised orbitals in a tetrahedral fashion. The C C bond length ¡s 154 pm. The structure extends in space and produces a rigid three-dimensional network of carbon atoms. In this structure, directional covalent bonds are present throughout the lattice. It is very difficult to break extended covalent bonding and, therefore diamond is the hardest substance on the earth.
(Or, structure of graphite orfulierene)
c) CCI4 cannot be hydrolysed because due to the absence of vacant d-orbitais carbon cannot increase its coordination number beyond four. Hence, it cannot accomodate the lone pair of electrons from oxygen atom of water molecule.
Silicates : Silicate is the general term given to solids with Si -O bonds. A large percentage of the earth’s crust consists of silicate minerals. Some of the important silicate minerals are quartz, asbestos, feldspar, mica, zeolites. The basic structural unit of silicates is silicate ion (SiO/-) in which Si atom is bonded to four O atoms in a tetrahedral fashion as shown below:
a) Diborane Or B2, H6
b) In diborane, the four-terminal hydrogen atoms and the two boron atoms lie in one plane. Above and below this plane, there are two bndging hydrogen atoms. The four-terminal B-H bonds are regular two-centre-two electron bonds while the two bridge (B-H-B) bonds are different and can be described in terms of three-centre-two electron bonds also refered to as banana bonds. Each B atom uses sfr hybrid orbitais for bonding. Out of the four sp3 hybrid orbitaIs on each B atom, one is without an electron.
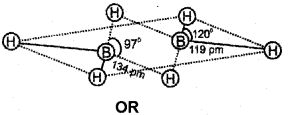
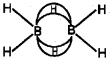
c) Boron compounds can act as Lewis acid because they are electron deficient. In boron compounds there are only six electrons around boron. Hence, they easily react with Lewis bases like NH3 to complete octet around boron.
Question 16.
a) CCI4 does not undergo hydrolysis but SiCI4 undergoes hydrolysis. Why? (Say – 2016)
b) Differentiate between silicates and silicons.
Answer:
a) CCI4 does not undergo hydrolysis because carbon atom cannot increase Its coordination number beyond four due to the absence of d-orbitais. It cannot accommodate the lone pair of electrons from oxygen atom of water molecule. Whereas SiCl4 can undergo hydrolysis because the central atom Si has vacant d-orbitais in its valence shell and ¡t can accommodate the lone pair of elecrons from oxygen of water molecule in its dorbital.
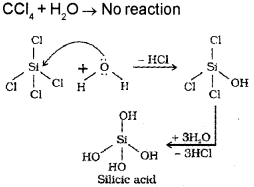
b) Silicones: These are a group of organosilicon polymers, which have (-R2SiO-) as a repeating unit.
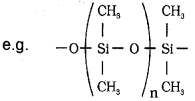
The starting materials for the manufacture of silicones are alkyl or aryl substituted silicon chlorides. Silicones being surrounded by nonpolar alkyl groups are water repelling in nature. They have high thermal stability, high dielectric strength and resistance to oxidation and chemicals.
Silicates: Silicate is the general term given to solids with Si -O bonds. A large percentage of the earths crust consists of silicate minerals. Some of the important silicate minerals are quartz, asbestos, feldspar, mica, zeolites. The basic structural unit of silicates is silicate ion (SiC44-) in which Si atom is bonded to tour O atoms in a tetrahedral fashion as shown below:
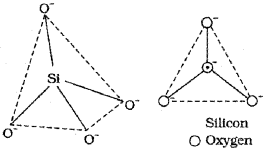
In silicates eitherthe discrete unit is present ora number of such units are joined together via corners by sharing 1, 2, 3 or 4 oxygen atoms per silicate units. When silicate units are linked together they form chain, ring, sheet or 3D structures. Negative charge on silicate structure is neutralised by positively charged metal ions. If all the four corners are shared with other tetrahedral units, 3D network is formed.
Question 17.
Borax is an important compound of Boron. (March – 2017)
a) The solution of borax is alkaline. Givea reason.
b) Giveanytwo usesof borax.
c) Diamond has co-valent bonding. Yet it has high melting point. Give a reason.
Answer:
a) Borax dissolves in water to give NaOH and H3BO3 (orthoboric acid).
Na2B4O7 + 7H2O → 2NaOH + 4H3BO3
Since NaOH is a strong base and H3BO3 is a weak acid the aqueous solution of borax is alkaline.
b)
- For the detection of transition metals by borax bead test
- Manufacture of heat resistant glasses (e.g. Pyrex), glass-wool and fibre glass.
- As a flux for soldering metals.
- For heat, scratch and stain resistant glazed coating to earthenwares.
- As constituent of medicinal soaps. (Any two uses required)
c. In diamond each carbon atom undergoes sp3 hybridisation and is linked to four other carbon atoms by using hybridised orbitals in tetrahedral fashion. The C-C bond length is 154 pm. The structure extends in space and produces a rigid three-dimensional network of carbon atoms. In this structure directional covalent bonds are present throughout the lattice. It is very difficult to break extended covalent bonding and, therefore diamond has high melting point and is the hardest substance on the earth.
We hope the Kerala Plus One Chemistry Chapter Wise Previous Questions Chapter 11 The p-Block Elements help you. If you have any query regarding Kerala Plus One Chemistry Chapter Wise Previous Questions Chapter 11 The p-Block Elements, drop a comment below and we will get back to you at the earliest.