What is the Biogeochemical Cycle
Biogenic elements (macro-, micro- & other elements) flow from the environment into and out of the plant in a cyclic manner.
This flow of nutrients from abiotic to biotic components of the ecosystem and vice-versa constitute the biogeochemical cycles.
Hydrological or Water Cycle :
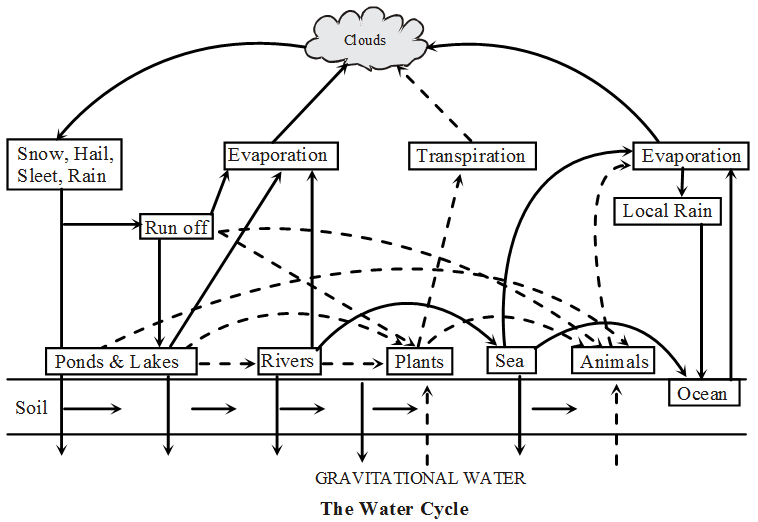
- Water on earth is cycled by two processes, evaporation and precipitation.
- The atmospheric precipitation occurs in the form of snow, hail or sleet etc. The run off water is finally collected in ocean through rivers.
- Some water remains solid in the form of snow which gradually melts and reaches the sea.
- Soil water is used by plants and most of it again reaches the atmosphere through transpiration.
- Animals consume water directly from water bodies & also the gravitational water.
- By evaporation, the water returns to atmosphere and cycle is repeated.
Carbon Cycle :
- CO2 is 0.03% in atmosphere, which is utilized by producers in photosynthesis for making food.
- From producers, it goes to consumers and then through decomposers into atmosphere.
- The producers, consumers & decomposers may be converted into fossil fuel (petrol, coal etc.) or form carbonate rock after death.
- By way of respiration the biotic component returns CO2 to atmosphere.
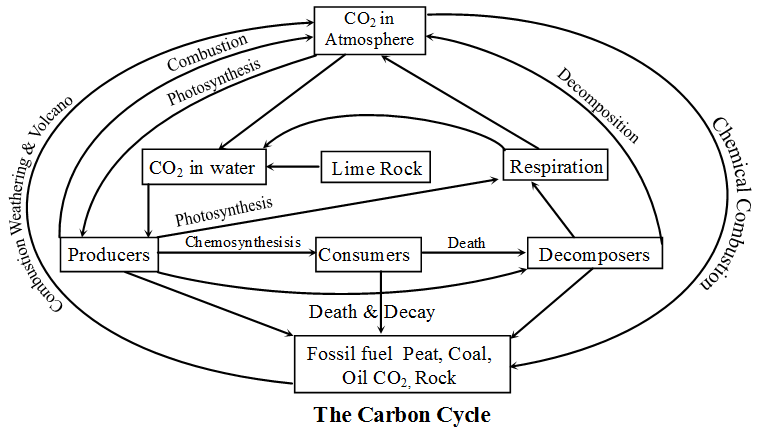
- CO2 may get dissolved in water. The lime rocks also contribute to CO2 in water. The aquatic producer use this CO2 for photosynthesis and return it by respiration.
- By combustion of fossil fuel & also by volcanic activity, CO2 is returned to the atmosphere.
Nitrogen Cycle :
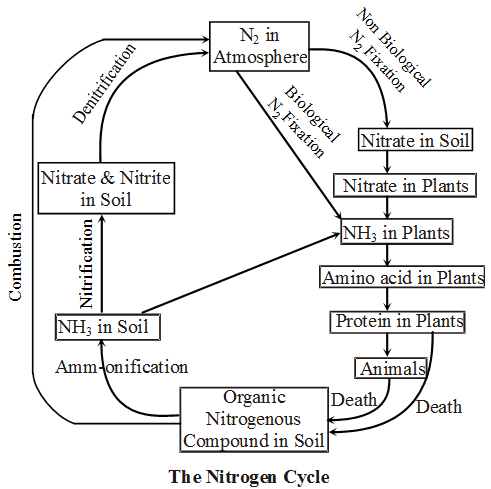
- The atmosphere is the source of N2 where it is about 79%. Plant cannot use N2 directly.
- In living organisms nitrogen is important constituent of protein and nucleic acid.
- The N2 cycle has five important steps –
- Nitrogen Fixation :
Conversion of N2 gas into its compounds like nitrates & nitrites is called N2 fixation. It is done either non-biologically by lightening or biologically by symbiotic or free- living bacteria. O2 is harmful for N2fixing bacteria. - Assimilation of Nitrogen :
N2 cannot be used by plants directly. They absorb it in the form of nitrate. Nitrate later on reduced to ammonia which provide amino (–NH2) group. It is important part of proteins. - Ammonification :
Dead plant & animal protein and their waste like urea & uric acid converted to ammonia by some ammonifying bacteria in soil. e.g. Bacillus mycoides, B. vulgaris & B. ramosus etc. - Nitrification :
Ammonia is converted into nitrite by Nitrosomonas bacteria, and Nitrobacter convert nitrite into nitrate. This nitrate again can be absorbed by plant & thus cycled back. - Denitrification :
Some denitrifying bacteria like Pseudomonas reduce nitrate into nitrogen gas in soil. This gas is again back to environment.
- Nitrogen Fixation :