What are the Uses of Electrolysis
Uses of Electrolysis
The process of electrolysis has many important industrial applications. Some of them are as follows:
- Electrolysis is used in industry for the production of many metals and non-metals (e.g., aluminium, magnesium, chlorine, and fluorine).
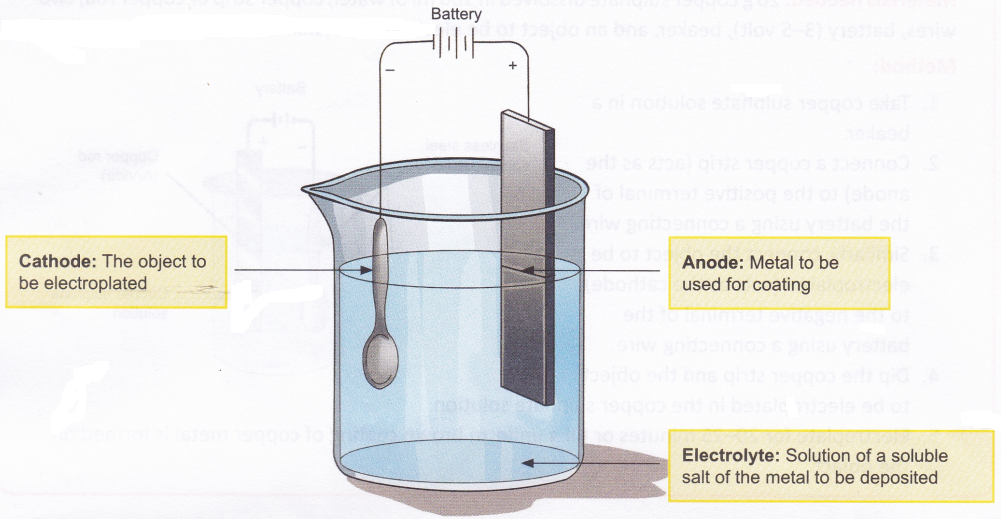
- Electrolysis is commonly employed for coating one metal with another. The method of coating one metal with another using an electric current is called electroplating. The general set-up used for electroplating an object is shown in Figure.
The method involved in electroplating is as follows:- The object to be coated is made the cathode.
- The metal to be deposited on the object is taken in the form of an electrode and made the anode.
- The electrolyte contains dissolved salts of the metal to be coated.
- Ions of the metal (which are positively charged) are attracted by the cathode and therefore move towards the object and get deposited on it.
Electroplating is done for many purposes. Here are a few examples.
- Metals that corrode easily are protected by coating them with a metal that does not corrode easily. Nickel and chromium are widely used in the automobile industry for coating.
- Electroplating is used for decoration. For example, cutlery, statues, and jewellery made of cheaper metals are coated with expensive metals like gold and silver to enhance their look.
- Electroplating is used in the manufacture of printed circuit boards (small circuits with components generally printed on a plastic board), which are used in TV, computer, etc.
Activity
Aim: To observe electroplating of copper on stainless steel.
Materials needed: 20 g copper sulphate dissolved in 100 ml of water, copper strip or copper rod, two wires, battery (3-5 volt), beaker, and an object to be electroplated (stainless steel spoon or a coin).
Method:
1. Take copper sulphate solution in a beaker.
2. Connect a copper strip (acts as the anode) to the positive terminal of the battery using a connecting wire.
3. Similarly, connect the object to be electroplated-(acts as the cathode) to the negative terminal of the battery using a connecting wire.
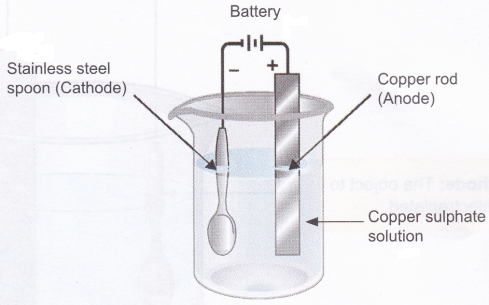
4. Dip the copper strip and the object to be electroplated in the copper sulphate solution.
5. Electroplate for 20-25 minutes or till a uniform brown coating of copper metal is formed on the object.
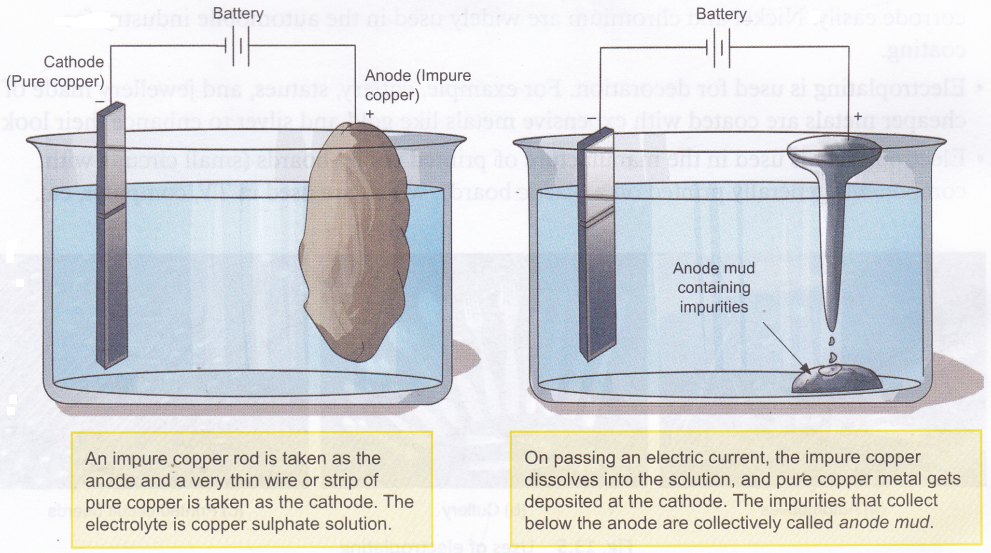
Electrolysis is used in the refining of impure metals. This method, called electrorefining, can be used to purify metals like copper, gold, and silver. Here is how the purification of copper is carried out.
Activity
Aim: To observe the deposition of copper using copper electrodes and copper sulphate solution
Materials needed: A battery, electrical wires, a thick copper strip, a thin copper wire (with jacket removed), copper sulphate solution, and a glass tumbler. The glass tumbler should be discarded after the experiment.
Method: Set up the experiment as shown in the figure. Wait for some time and see what happens to the cathode.
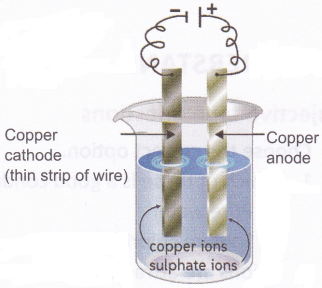
Observation: You will observe that the pure copper cathode gets a coating of copper.